Probiotics for Antibiotic Recovery: Science-Backed Strategies to Restore Gut Balance
Key Takeaways
- Antibiotics disrupt gut microbiota composition, reducing bacterial diversity and increasing susceptibility to digestive complications
- Specific probiotic strains—particularly Lactobacillus rhamnosus GG and Saccharomyces boulardii—reduce antibiotic-associated diarrhea risk by 51-71%
- Soil-based probiotic organisms (Bacillus species) form protective spores that survive stomach acid and antibiotic exposure
- Multi-strain formulations may provide broader microbiome support, though single well-researched strains remain effective
- Optimal timing: take probiotics at least 2 hours before or after antibiotics, continue for 2 weeks post-treatment
Antibiotic therapy, while essential for treating bacterial infections, creates an unavoidable biological paradox: these medications cannot distinguish between pathogenic bacteria and the beneficial microorganisms that maintain digestive health. This indiscriminate effect leads to what researchers term "antibiotic-associated dysbiosis"—a disruption of the gut microbiome that affects up to 35% of antibiotic users.[1]
The question facing both clinicians and patients is not whether antibiotics affect the microbiome (the evidence is unequivocal), but rather how to mitigate this disruption through evidence-based probiotic supplementation. Recent systematic reviews and meta-analyses provide increasingly clear guidance on which strains work, at what dosages, and under what circumstances.[2,3]
Understanding Antibiotic Impact on Gut Microbiota
Broad-spectrum antibiotics—the most commonly prescribed class—reduce microbial diversity in the gut by targeting both gram-positive and gram-negative bacteria. This reduction in diversity has been documented to persist for weeks to months after antibiotic cessation, with some studies showing alterations lasting up to two years.[4]
The clinical consequences extend beyond transient digestive discomfort. Antibiotic-induced dysbiosis has been associated with increased colonization by opportunistic pathogens including Clostridioides difficile, altered metabolite production (particularly short-chain fatty acids that support intestinal barrier function), and increased expression of antibiotic resistance genes within the gut resistome.[5]
Research from 2024 confirms that antibiotic use, particularly in childhood, correlates with increased lifetime risk of immune-mediated diseases including allergies and inflammatory bowel conditions, suggesting the microbiome disruption has immunological implications that extend far beyond the gastrointestinal tract.[6]
Evidence-Based Probiotic Strains for Antibiotic Support
Not all probiotics demonstrate equal efficacy during antibiotic therapy. Strain-specific research has identified several organisms with robust clinical evidence supporting their use alongside antibiotics.
Lactobacillus rhamnosus GG
A 2015 systematic review analyzing data from 1,497 patients found that L. rhamnosus GG reduced antibiotic-associated diarrhea incidence from 22.4% in controls to 12.3% in supplemented groups—a 45% relative risk reduction.[7] The protective effect was particularly pronounced in pediatric populations, where supplementation with 1-2 × 1010 CFU daily reduced diarrhea incidence by 71%.[7]
The strain's mechanisms include production of antimicrobial peptides that inhibit pathogen colonization, modulation of intestinal permeability through upregulation of tight junction proteins, and stimulation of secretory IgA production.[8]
Saccharomyces boulardii
This non-pathogenic yeast demonstrates unique advantages during antibiotic therapy: as a eukaryotic organism, it remains unaffected by antibacterial agents. A meta-analysis of 21 randomized controlled trials (4,780 participants) found that S. boulardii reduced antibiotic-associated diarrhea risk from 18.7% to 8.5%—a 53% relative risk reduction with a number-needed-to-treat of 10.[9]
The organism produces a 54-kDa serine protease that directly degrades C. difficile toxins while simultaneously destroying colonic receptor sites for toxin binding, providing dual-mechanism protection against this common antibiotic-associated complication.[10]
Multi-Strain Formulations
A 2024 multicenter trial evaluated a high-dose multi-strain probiotic containing Lactobacillus spp., Bifidobacterium spp., Bacillus coagulans, and S. boulardii in adults receiving antibiotics. The probiotic group experienced significantly reduced antibiotic-associated diarrhea (7.3% vs 28.4% in placebo), with excellent safety profiles across all participants.[11]
While some research questions whether multi-strain formulations offer advantages over single well-researched strains, a systematic review comparing 65 randomized controlled trials found that in most cases, properly dosed single strains performed equivalently to mixtures.[12] However, for certain applications—particularly when seeking to restore overall microbiome diversity rather than prevent specific complications—multi-strain approaches may provide complementary benefits.[13]
Soil-Based Organisms: Spore-Forming Resilience
Bacillus species represent a distinct category of probiotics characterized by spore formation—a survival mechanism that confers remarkable stability under adverse conditions including gastric acid, bile salts, and antibiotic exposure.[14]
These spore-forming probiotics survive processing, storage, and gastrointestinal transit more reliably than vegetative bacterial cells. Research demonstrates that Bacillus spores germinate specifically in the small intestine when exposed to nutrients and conducive pH conditions, then resporulate before excretion—providing transient colonization that supports the resident microbiome without permanent engraftment.[15]
Species including B. coagulans, B. subtilis, B. clausii, B. licheniformis, and B. pumilus have demonstrated clinical efficacy across various digestive conditions. Notably, B. clausii strains possess natural antibiotic resistance mechanisms, making them particularly suitable for concurrent administration with antibiotics.[16]
A 2024 scientific review emphasized that Bacillus species produce antimicrobial compounds, digestive enzymes (including amylases, proteases, and lipases), and various vitamins—providing functional benefits beyond simple bacterial competition.[17]
Practical Application: Timing and Dosage
Probiotic efficacy depends critically on proper administration timing. Research supports initiating probiotic supplementation within the first two days of antibiotic therapy and continuing for at least two weeks after antibiotic completion.[18]
To maximize survival and colonization, probiotics should be administered at least 2 hours before or after antibiotic doses. This separation reduces direct antibiotic exposure to probiotic organisms while maintaining therapeutic antibiotic levels.[19]
Effective dosages vary by strain but generally range from 5 × 109 to 1 × 1010 colony-forming units (CFU) daily. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition recommends minimum daily doses of 5 billion CFU for clinically proven strains.[20]
Supporting Gut Recovery Beyond Probiotics
While probiotics address bacterial repletion, comprehensive gut recovery requires attention to the broader intestinal ecosystem. Prebiotic fibers—particularly fermentable fibers that resist upper gastrointestinal digestion—selectively nourish beneficial bacteria while discouraging pathogen overgrowth.[21]
Dietary strategies supporting microbiome recovery include:
- Fermented foods containing live cultures (yogurt, kefir, kimchi, sauerkraut) provide additional probiotic organisms alongside fermentation-derived metabolites
- Resistant starches from cooked and cooled potatoes, rice, and legumes serve as substrates for butyrate-producing bacteria
- Polyphenol-rich foods (berries, green tea, dark chocolate) undergo microbial metabolism to produce bioactive compounds supporting gut barrier function
- Prebiotic fibers including inulin, fructooligosaccharides, and galactooligosaccharides preferentially stimulate beneficial bacterial growth
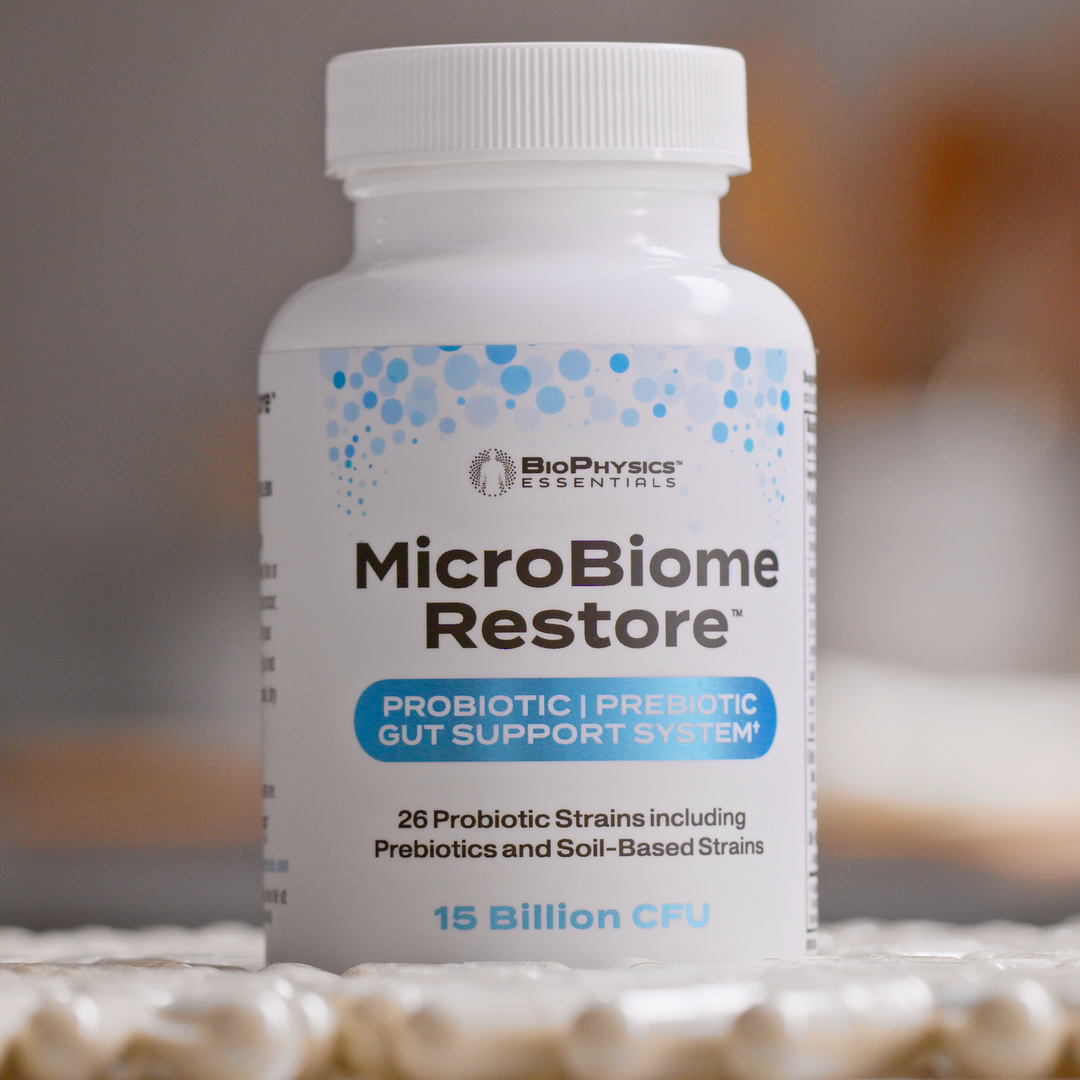
Example Formulation: MicroBiome Restore
For those seeking a comprehensive approach, MicroBiome Restore represents an example of modern multi-strain probiotic design. The formulation combines 26 probiotic strains (15 billion CFU) with 9 certified organic prebiotics, including five Bacillus soil-based organisms (B. coagulans, B. clausii, B. licheniformis, B. pumilus, B. subtilis) alongside lactic acid-producing Lactobacillus and Bifidobacterium species.
The formulation employs fermented pullulan capsules with inherent prebiotic properties, contains no fillers (no magnesium stearate, silicon dioxide, or microcrystalline cellulose), and remains shelf-stable without refrigeration—addressing common concerns about probiotic viability and formulation purity.
While this represents one evidence-informed approach to probiotic supplementation during antibiotics, the fundamental principles—strain specificity, adequate dosing, proper timing, and comprehensive prebiotic support—apply regardless of the specific product chosen.
Safety Considerations and Individual Variation
Probiotic safety in healthy populations is well-established, with adverse events in clinical trials showing no statistical difference from placebo.[22] However, specific populations require additional consideration:
- Immunocompromised individuals should consult healthcare providers before probiotic use, as rare cases of bacteremia and fungemia have been reported in severely immunosuppressed patients[23]
- Critically ill patients with compromised intestinal barrier function may face increased translocation risk
- Individuals with central venous catheters should exercise caution with fungal probiotics like S. boulardii
Individual microbiome composition influences probiotic response. Research demonstrates that probiotics colonize more readily in "colonization-permissive" individuals, while others show resistance to microbial engraftment.[24] This person-specific variation suggests that microbiome-based therapeutics may require individualized approaches in the future, though current evidence supports empiric use of well-researched strains in most populations.
The Broader Context: Antibiotic Stewardship
While probiotics mitigate antibiotic-associated complications, the fundamental approach to preserving microbiome health involves judicious antibiotic use. The American Gastroenterological Association's 2024 guidelines emphasize that antibiotic prescription should balance bacterial infection treatment against microbiome disruption risks.[25]
When antibiotics are medically necessary, probiotic co-administration represents an evidence-based strategy to minimize collateral microbiome damage. A 2024 review of microbiome-targeting therapeutics notes that while fecal microbiota transplantation and next-generation probiotics show promise for specific conditions, traditional probiotics remain the most accessible and evidence-supported intervention for antibiotic-associated dysbiosis.[26]
Conclusion: Integrating Evidence into Practice
The relationship between antibiotics and the gut microbiome represents a complex biological trade-off: infection control at the cost of collateral microbiome disruption. Probiotic supplementation, particularly with well-researched strains like L. rhamnosus GG, S. boulardii, and specific Bacillus species, provides measurable risk reduction for antibiotic-associated complications.
Key implementation points include:
- Initiate probiotics within 48 hours of antibiotic commencement
- Select strain-specific products with published clinical evidence
- Administer at least 2 hours separated from antibiotic doses
- Continue supplementation for 2 weeks post-antibiotic completion
- Support probiotic efficacy with prebiotic fiber intake and fermented foods
- Consult healthcare providers if immunocompromised or critically ill
As microbiome science advances, the integration of probiotics, prebiotics, dietary modification, and judicious antibiotic stewardship represents a comprehensive framework for maintaining gastrointestinal health during necessary antimicrobial therapy.
For additional guidance on optimal probiotic timing, single vs. multi-strain selection, and evidence-based probiotic selection criteria, consult our comprehensive gut health resources.


Share and get 15% off!
Simply share this product on one of the following social networks and you will unlock 15% off!