Hidden Fillers in Your Probiotics: What You're Really Swallowing
Introduction
You carefully research probiotic strains, check CFU counts, and compare prices—but have you looked at what else is in that capsule? While consumers focus on the beneficial bacteria listed on the front label, hidden fillers and excipients lurking in the "Other Ingredients" section may be quietly undermining your gut health goals. From microcrystalline cellulose to titanium dioxide, these manufacturing additives aren't just inert space-fillers—emerging research reveals they can alter your microbiome, trigger inflammation, and even affect the gut-brain axis.
This science-based guide examines what the peer-reviewed research actually says about common probiotic fillers, which ingredients pose legitimate concerns, and how to decode supplement labels to make informed choices that maximize your investment in gut health.
Key Takeaways
- ✓ Not all fillers are harmful: Research distinguishes between problematic additives (titanium dioxide, maltodextrin) and relatively safe ones (magnesium stearate, gelatin, microcrystalline cellulose)
- ✓ Titanium dioxide alters gut microbiota: Systematic reviews show it reduces beneficial Lactobacillus, increases inflammatory markers, and depletes protective short-chain fatty acids
- ✓ Maltodextrin undermines probiotic function: Studies demonstrate it breaks down intestinal mucus, enhances pathogenic biofilms, and correlates with inflammatory bowel disease
- ✓ Microcrystalline cellulose may actually help: Clinical trials found high-dose MCC reduces inflammation markers by 39% and supports gut barrier function
- ✓ Magnesium stearate is largely misunderstood: Despite consumer concerns, comprehensive safety testing shows no genotoxicity or microbiome disruption at supplement doses
- ✓ Gelatin capsules protect probiotics: Research confirms gelatin coating increases beneficial bacteria survival through stomach acid by 47%
- ✓ The regulatory gap enables problematic practices: FDA's post-market approach allows manufacturers to self-certify ingredient safety without pre-approval
- ✓ Label reading requires specific knowledge: Understanding terminology like "flow agents," "bulking agents," and recognizing alternative ingredient names is essential
Understanding Supplement Fillers: What They Are and Why They're Used
The Manufacturing Reality Behind "Other Ingredients"
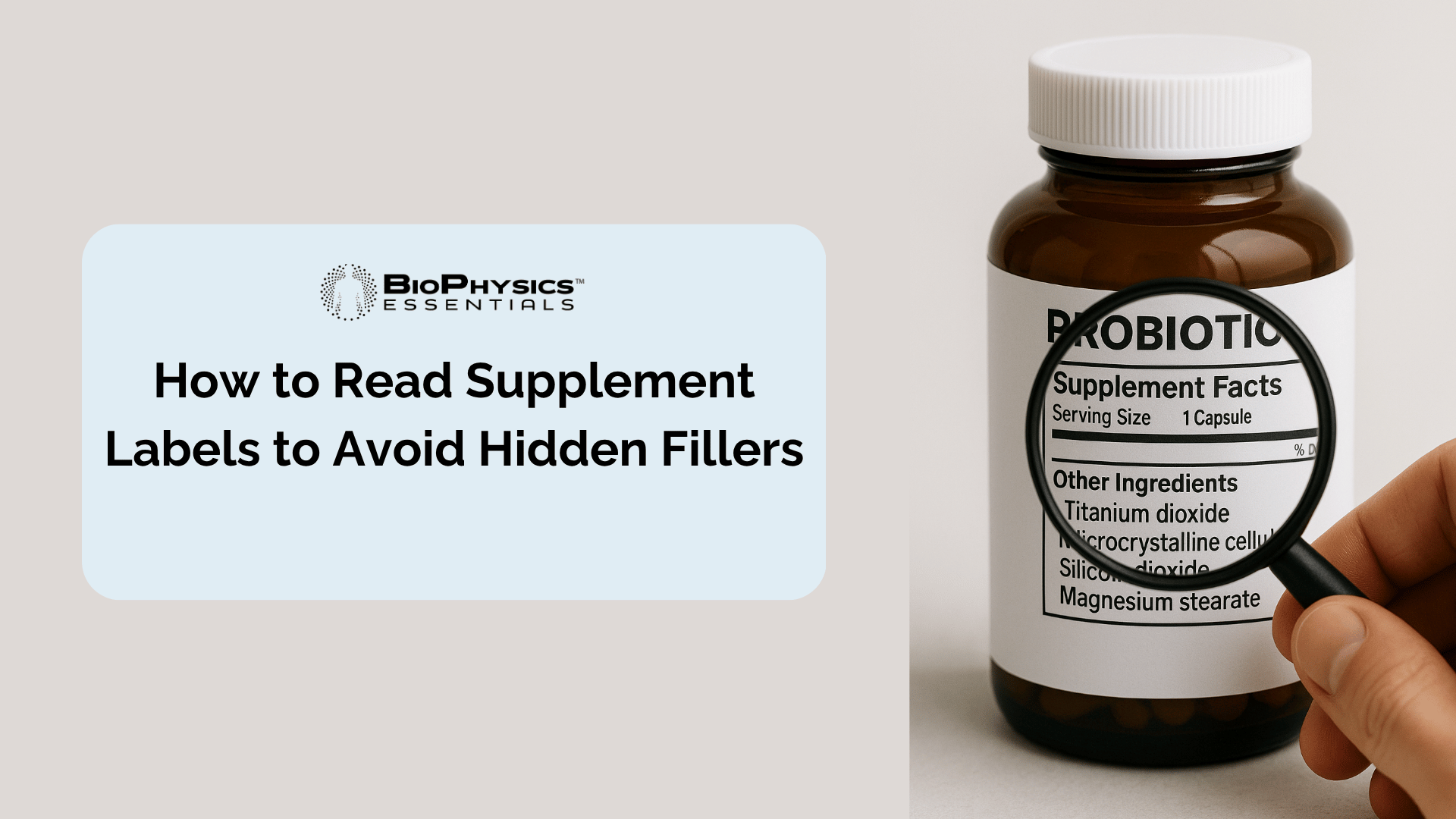
Open any supplement bottle and flip to the back label. Below the "Supplement Facts" panel, you'll find a section called "Other Ingredients"—and this is where fillers hide in plain sight. But what exactly are these additives, and why do manufacturers use them?
Fillers serve several manufacturing functions: Flow agents (like magnesium stearate and silicon dioxide) prevent ingredients from sticking to machinery during encapsulation. Bulking agents (like microcrystalline cellulose and maltodextrin) add volume when active ingredients are microscopically small—without them, you'd be swallowing nearly-empty capsules. Binders hold compressed tablets together, while disintegrants help capsules break down for absorption.
The problem isn't necessarily that these ingredients exist—it's that manufacturers often prioritize production efficiency over consumer health. What's cheapest and fastest to manufacture isn't always what's best for your gut. Some fillers serve legitimate functional purposes, while others are purely cost-cutting measures with documented biological effects.
Common Probiotic Fillers: The Complete List
The most frequently encountered fillers in probiotic supplements include:
- Microcrystalline cellulose (MCC): Plant-derived cellulose used for bulking
- Magnesium stearate: A salt combining magnesium and stearic acid, used as a flow agent
- Silicon dioxide (silica): An anti-caking agent preventing clumping
- Titanium dioxide: A whitening agent and colorant
- Maltodextrin: A starch-derived powder used as a carrier for probiotics
- Gelatin or hypromellose: Capsule shell materials
- Rice flour or rice bran: Natural bulking agents
- Stearic acid: A fatty acid similar to magnesium stearate
Understanding which of these ingredients have documented health impacts—and which are relatively benign—requires examining the peer-reviewed research rather than relying on marketing claims or internet fearmongering.
The Science on Problematic Fillers: What Research Actually Shows
Titanium Dioxide: The Filler Europe Banned
If any filler deserves concern, it's titanium dioxide (TiO2). This white pigment appears in supplements purely for cosmetic reasons—to make capsules and tablets bright white. It provides zero nutritional or functional benefit, yet systematic reviews of animal studies reveal concerning effects on gut health.
Research published in the International Journal of Environmental Research and Public Health analyzed 18 animal studies and found that titanium dioxide exposure causes significant variations in bacterial species abundance, particularly reducing beneficial Lactobacillus populations while enriching Proteobacteria—a pattern associated with inflammatory bowel disease and obesity[1]. The studies documented that TiO2 reduces production of protective short-chain fatty acids, depletes mucus that shields the intestinal lining, and increases inflammatory biomarkers including IL-1, IL-6, IL-8, and TNF-α[1].
The evidence proved compelling enough that the EU decided to ban titanium dioxide in European food products in 2022. The European Food Safety Authority concluded they couldn't exclude concerns about genotoxicity and potential carcinogenic effects. Yet in the United States, titanium dioxide remains "Generally Recognized as Safe" and continues appearing in supplements—including some probiotics designed to support gut health.
The irony is stark: consumers take probiotics to increase beneficial Lactobacillus bacteria, while the titanium dioxide in their capsule may be reducing those same bacterial populations.
Maltodextrin: The Carrier That Undermines Probiotic Function
Maltodextrin represents an even more insidious problem because it's frequently used as the carrier matrix for freeze-dried probiotic bacteria. This starch-derived powder keeps bacterial strains separated during manufacturing—but research reveals it may actively work against gut health.
A study published in Cellular and Molecular Gastroenterology and Hepatology found that maltodextrin consumption significantly increases endoplasmic reticulum stress in gut epithelial cells, leading to reduced MUC2 mucus production[2]. This protective mucus layer normally separates bacteria from directly contacting the intestinal epithelium—its breakdown creates vulnerability to infection and inflammation.
Additional research in Gut Microbes demonstrated that maltodextrin markedly enhances E. coli biofilm formation and increases bacterial adhesion to intestinal epithelial cells[3]. When mice consumed maltodextrin-supplemented water, researchers observed breakdown of the anti-microbial mucous layer. When these mice were given oral Salmonella infection, maltodextrin-fed animals showed significantly increased bacterial loads compared to controls[3].
Perhaps most troubling, a 2022 systematic review examining 70 randomized controlled trials concluded that maltodextrin cannot be considered an inert placebo—it has significant physiological effects on gut function[4]. The researchers noted an epidemiological correlation between increasing dietary maltodextrin prevalence and the dramatic rise in Crohn's disease incidence over recent decades.
Using maltodextrin as a probiotic carrier is like building a house on a foundation designed to erode—the carrier actively undermines what the probiotics are trying to accomplish.
Silicon Dioxide: Size Matters More Than We Knew
Silicon dioxide (silica) occupies more ambiguous territory. While large-particle silica appears relatively safe, research reveals that nanoparticle-sized silica—increasingly common in modern food-grade additives—behaves very differently in biological systems.
A study published in Environment International found that 10-nm-sized silica nanoparticles significantly exacerbated dextran sulfate sodium-induced colitis in mice, while 30-nm particles showed no such effect[5]. This size-dependent toxicity suggests the form of silica matters enormously—yet supplement labels never specify particle sizes.
Research published in Journal of Nanobiotechnology documented that silicon dioxide nanoparticles induce neurobehavioral impairments through disruption of the microbiota-gut-brain axis, affecting spatial learning, memory, and locomotor function in animal models[6]. Additional studies confirmed that food-grade silica nanoparticles cause inflammatory damage to the intestine and lead to changes in gut microbiota composition, particularly affecting mucus-associated bacteria[5].
The European Food Safety Authority's 2018 re-evaluation of silicon dioxide concluded they could not establish an acceptable daily intake due to insufficient nanoparticle characterization and toxicity data[7]. They specifically recommended reformulation to eliminate nano-sized particles—advice many supplement manufacturers have not heeded.
For consumers, the challenge is that "silicon dioxide" on a label provides no information about particle size, leaving uncertainty about whether you're getting the apparently-safe larger particles or the potentially-problematic nanoforms.
The Surprising Science on "Acceptable" Fillers
Microcrystalline Cellulose: When "Safe" Still Means "Wasted Space"
Microcrystalline cellulose often gets defended as the "good" filler—and to be fair, the research shows it's not harmful. This plant-derived cellulose serves as a bulking agent, and unlike maltodextrin or titanium dioxide, clinical studies actually suggest MCC may provide anti-inflammatory benefits.
A six-week randomized controlled trial published in Microbiome examined high-dose microcrystalline cellulose supplementation (25-35g daily) in 16 healthy adults. The results showed MCC reduced the inflammatory marker TNF-α by 7% (p=0.004) and decreased fecal calprotectin—a marker of intestinal inflammation—by 39% (p=0.004)[8]. Supporting research in Journal of Agricultural and Food Chemistry confirmed that MCC exhibited anti-inflammatory activities and enhanced gut barrier function[9].
But here's the critical question: if MCC shows benefits at 25-35 grams daily, why are manufacturers using it as a filler in tiny amounts that provide no therapeutic value? The answer is simple—manufacturing convenience, not your health. That capsule space could hold more probiotic strains, additional prebiotic fibers, or complementary botanicals that enhance gut function. Instead, it's filled with an inert bulking agent because it makes production easier.
The fact that MCC isn't actively harmful misses the point entirely. Every milligram of space in a capsule represents an opportunity to support your gut health. Wasting that space on manufacturing aids—even "safe" ones—means settling for a product that could be better.
Magnesium Stearate: Safe Doesn't Mean Necessary
Magnesium stearate has become perhaps the most controversial supplement filler, with internet claims ranging from "toxic" to "causes cancer." The peer-reviewed research tells a different story—but one that still doesn't justify its use.
Comprehensive genotoxicity testing published in Toxicology Reports found no mutagenic response in bacterial reverse mutation assays at concentrations up to 5,000 μg/plate, no chromosomal aberrations in mammalian cells, and no micronuclei formation in mouse bone marrow[10]. Research on dietary magnesium's effects on gut microbiota found that only extremely high magnesium supplementation (6,000 mg/kg diet) altered microbiota composition—100-1,000 times higher than supplement levels[11].
So magnesium stearate appears safe. But here's what matters: it provides absolutely zero benefit to you as a consumer. Its only purpose is making manufacturing faster and cheaper. It prevents ingredients from sticking to machinery and enables higher production speeds—benefits that accrue entirely to manufacturers, not to your gut health.
The legitimate concern about magnesium stearate relates to dissolution time—some studies show capsules containing it take longer to break down. For probiotics specifically, any delay in capsule breakdown means fewer live bacteria reaching your intestines. But even if we ignore that concern, the fundamental question remains: Why should you swallow ingredients that serve only the manufacturer's bottom line?
Premium manufacturers prove every day that formulation without flow agents is possible. It requires specialized equipment, slower production, and more careful engineering—but it's absolutely achievable. Accepting magnesium stearate means settling for "good enough" when "optimal" is within reach.
Gelatin Capsules: An Unexpected Probiotic Protector
While vegetarians and vegans rightly seek plant-based alternatives, research on gelatin capsules reveals they may actually enhance probiotic survival and delivery.
A study published in LWT - Food Science and Technology examined Lactobacillus paracasei survival under simulated gastrointestinal conditions. The research found that gelatin coating significantly improved bacterial viability, with up to 47% increased survival compared to free cells after exposure to biliary salts[12]. During 35 days of refrigerated storage, microencapsulated cells with gelatin coating remained stable while free cells lost 28-35% viability. The gelatin coating also protected probiotics during pH 1.5 exposure simulating stomach acid.
This protection mechanism explains why many premium probiotic formulations specifically use gelatin despite consumer preference for plant-based options. The trade-off between philosophical preference and functional efficacy becomes a personal decision—though the emergence of advanced plant-based capsule technologies (like fermented tapioca) increasingly offers the best of both worlds.
The Regulatory Gap: Why Problematic Fillers Remain Legal
FDA's Post-Market Approach Enables Self-Certification
Understanding why supplements with questionable fillers remain on store shelves requires understanding the regulatory landscape—or lack thereof. Unlike pharmaceuticals, which require extensive pre-market approval, dietary supplements operate under a post-market enforcement model.
The FDA's authority over supplements comes from the Dietary Supplement Health and Education Act of 1994, which places the burden of proof on the FDA to demonstrate harm rather than requiring manufacturers to prove safety before sale. Manufacturers self-certify compliance with 21 CFR Part 111 (current Good Manufacturing Practice requirements) and self-determine Generally Recognized as Safe (GRAS) status for ingredients.
This creates an accountability gap where companies can add ingredients to foods via self-certified GRAS status, then use those same ingredients in supplements without New Dietary Ingredient notifications. Environmental health organizations have documented that supplement makers "routinely and systematically" exploit this loophole.
All ingredients must appear on labels under "Other Ingredients," but manufacturers face no requirement to disclose quantities for inactive ingredients—only listing them in descending order by weight. The proprietary blend loophole allows companies to disclose total blend weight without revealing individual ingredient amounts, hiding exactly how much consumers receive of each component.
Post-Market Enforcement Proves Ineffective
Even when the FDA identifies problems, enforcement remains limited. A 2022 study published in JAMA Internal Medicine examined FDA warning letters for adulterated supplements and found troubling patterns: only 3% of implicated products were recalled after warnings, 29% remained available for purchase six years later, and most still contained the prohibited ingredients they'd been warned about.
This enforcement gap exists because the FDA operates with limited resources and relies primarily on voluntary recalls. Without mandatory pre-market approval, problematic ingredients can reach consumers for years before action occurs—if action occurs at all.
Third-Party Certification Fills the Gap
Given regulatory limitations, third-party certifications provide the most reliable quality assurance. However, understanding what different certifications actually test is crucial:
USP Verified confirms identity, purity, strength, and dissolution through independent testing with annual facility audits and ongoing off-shelf product testing. NSF International offers multiple programs: NSF Contents Tested verifies ingredients match label claims, NSF Certified for Sport tests for 280+ banned athletic substances, and NSF GMP Registered audits manufacturing facilities only. ConsumerLab.com independently purchases retail products for testing and publishes results publicly—they're not paid by manufacturers to test their products.
The existence of these independent verification systems underscores the inadequacy of government oversight. Consumers who want assurance their supplements contain what labels claim—and don't contain harmful adulterants—must look beyond FDA registration to third-party verification.
How to Read Probiotic Labels Like an Expert
Decoding the Supplement Facts Panel
Reading probiotic labels requires understanding both what information must be disclosed and what manufacturers can legally hide. The Supplement Facts panel differs fundamentally from Nutrition Facts labels on conventional foods, with different disclosure requirements that can obscure critical information.
For probiotics specifically, the CFU count (Colony Forming Units) represents the key efficacy measure. However, consumers must verify whether guarantees apply "at end of shelf life" or merely "at time of manufacture." Products listing CFUs only at manufacturing may contain minimal viable bacteria by expiration date—the probiotics died during storage, but the label remains technically accurate.
Quality probiotic labels provide:
- End-of-shelf-life CFU guarantees: Ensuring viable bacteria when you actually take the supplement
- Complete strain identification: Including genus, species, AND strain designation (e.g., "Lactobacillus acidophilus LA-14" not just "Lactobacillus acidophilus")
- Storage requirements: Clear instructions about refrigeration if needed
- Complete ingredient disclosure: Full listing of all components including "other ingredients"
- Expiration date: Mandatory for probiotics due to viability degradation
Understanding Filler Terminology
Manufacturers use technical terminology that obscures the presence of fillers from casual consumers. Recognizing these categories helps decode what's actually in your capsule:
Flow Agents/Glidants: Magnesium stearate, silicon dioxide, talc—prevent powder clumping during manufacturing. Bulking Agents/Fillers: Microcrystalline cellulose, rice flour, maltodextrin—add volume when active ingredients are microscopic. Binders: Cellulose, starch, gelatin—hold tablets together structurally. Disintegrants: Croscarmellose sodium—help capsules break down for absorption.
Each category serves manufacturing functions, but quality varies dramatically. Premium manufacturers minimize quantities while maintaining functionality—inferior products use excessive amounts because these ingredients cost less than active components.
Recognizing Alternative Names for Common Fillers
Fillers hide behind multiple names that obscure their presence. Being able to recognize these alternatives is essential for making informed choices:
Cellulose appears as: Microcrystalline cellulose, methylcellulose, hydroxypropyl methylcellulose (HPMC), carboxymethylcellulose, or simply "plant cellulose." Magnesium stearate may be listed as: Stearic acid or calcium stearate. Sugar-based fillers include: Maltodextrin, dextrose, lactose, sorbitol, xylitol, and modified food starch. Colorants use chemical names: Titanium dioxide, FD&C Yellow No. 5, or other synthetic dye designations.
The more comfortable you become with these alternative names, the harder it becomes for marketing to obscure what's actually in the product.
Eight Red Flags That Signal Inferior Probiotic Quality
Warning Signs Every Consumer Should Recognize
Certain characteristics reliably indicate lower-quality probiotic formulations. Learning to spot these red flags helps you avoid wasting money on supplements unlikely to deliver meaningful benefits:
1. Proprietary Blends Without Individual Amounts
When labels list "Probiotic Blend: 100 billion CFU" without specifying how much of each strain, manufacturers hide that most CFUs come from cheap, less-effective strains while expensive, well-researched strains appear in trivial amounts. Transparency requires individual strain quantities.
2. CFU Counts Only "At Time of Manufacture"
This language reveals that manufacturers don't guarantee bacteria will be alive when you take the supplement. Reputable brands guarantee potency through expiration date because they use stabilization technologies and overage formulation to account for natural die-off.
3. Vague Strain Identification
Listing "Lactobacillus acidophilus" without strain designation (like LA-14) signals that manufacturers use generic, possibly less-effective bacteria. Clinical research identifies probiotics by specific strains because different strains of the same species can have dramatically different effects. Vague identification suggests manufacturers don't know—or don't want you to know—which strains they're using.
4. Excessive Additive Lists
Quality supplements typically contain fewer than 5-7 inactive ingredients with clear functional justification for each. When "Other Ingredients" lists exceed the active ingredient section, it signals cost-cutting through cheap fillers rather than investment in premium active components.
5. No Expiration Date on Probiotics
Live bacteria degrade over time—this is biological reality. Supplements without expiration dates either don't contain live bacteria (possibly using killed bacteria or spore forms with indefinite shelf life) or don't conduct stability testing to verify how long bacteria remain viable.
6. Absence of Third-Party Certification Seals
While not mandatory, third-party verification (USP, NSF, ConsumerLab) signals manufacturers have invested in independent quality verification. Absence doesn't prove poor quality, but presence provides assurance beyond manufacturer claims.
7. Disease Treatment Claims
FDA regulations prohibit supplements from claiming to treat, cure, or prevent specific diseases. When labels make such claims, they reveal either regulatory ignorance or willingness to violate regulations—both concerning qualities in supplement manufacturers.
8. "100% Natural" Claims on Products Containing Synthetic Ingredients
When marketing emphasizes "natural" or "clean label" but the ingredient list includes magnesium stearate, silicon dioxide, or titanium dioxide, it demonstrates deceptive marketing. These additives, while approved, aren't "natural" in any meaningful sense.
The Gummy Vitamin Exception
Gummy probiotics deserve special mention because their format creates unique quality challenges. Gummy vitamins rarely earn third-party certification, contain excessive added sugars that feed pathogenic bacteria, and show notoriously inconsistent potency—testing by independent labs has found gummy supplements ranging from 50-250% of labeled amounts for various nutrients.
For probiotics specifically, the heat and moisture required for gummy manufacturing creates hostile conditions for live bacteria. Most gummy probiotics either contain spore-based organisms (which survive better but may be less effective) or rely on heavy overages with the expectation that most bacteria will die during processing.
The Clean Label Movement: Market-Driven Change
Consumer Demand Shapes Industry Practices
Given the regulatory limitations on supplement oversight, market pressure from informed consumers represents the most powerful force driving formulation improvements. Consumer research data reveals that 81% of shoppers consider clean label important when purchasing supplements, and 67% of global consumers are influenced by clean label terminology on packaging.
The dietary supplements market overall projects growth to $306-454 billion by 2033-2035, with clean label ingredients specifically growing faster at the market's leading edge. Plant-based supplements show 9.81% compound annual growth rate through 2030—faster than the overall supplement market—driven by transparency demands from Millennial and Gen Z consumers who research ingredients before purchasing.
What "Clean Label" Actually Means (and Doesn't Mean)
Unlike "organic" or "non-GMO," which have legal definitions and certification standards, "clean label" remains undefined by any regulatory body. This ambiguity creates opportunity for greenwashing—products marketed as "clean" despite containing questionable ingredients.
In practice, clean label generally refers to:
- Minimal processing and recognizable ingredients
- Absence of artificial colors, flavors, and preservatives
- No synthetic additives when natural alternatives exist
- Transparent sourcing and manufacturing practices
- Simple, short ingredient lists consumers can understand
However, manufacturers can slap "clean label" on products containing silicon dioxide or magnesium stearate because these ingredients are technically approved. True clean label requires examining the actual ingredient list, not accepting marketing claims at face value.
The Economic Reality Behind Filler Use
Understanding why manufacturers use fillers requires acknowledging economic pressures. Fillers reduce costs through multiple mechanisms: they allow faster production speeds (flow agents prevent equipment jams), require less active ingredient per capsule (bulking agents fill space), and enable use of standard manufacturing equipment rather than specialized probiotic handling systems.
For large-scale manufacturers producing thousands of bottles daily across multiple product lines, these efficiencies translate to significant cost savings. The question becomes whether those savings benefit consumers through lower prices or simply increase profit margins while potentially compromising efficacy.
Premium manufacturers who eliminate unnecessary fillers typically invest in specialized equipment, accept slower production speeds, and formulate with overages to ensure end-of-shelf-life potency. These investments increase production costs—but for consumers prioritizing quality over price, the trade-off may be worthwhile.
When Filler-Free Formulations Matter Most
Individual Sensitivity and Vulnerability
While avoiding problematic fillers benefits everyone, certain situations make filler-free formulations especially critical. Research on titanium dioxide and maltodextrin shows pre-existing inflammation amplifies their negative effects—those with the most to gain from probiotics may be most vulnerable to filler interference.
Existing gut inflammation or inflammatory bowel disease: Studies demonstrate silica nanoparticles and titanium dioxide specifically exacerbate intestinal inflammation. For individuals with Crohn's disease, ulcerative colitis, or other inflammatory conditions, fillers documented to trigger inflammation directly counteract therapeutic goals.
Autoimmune conditions: Research showing silica alters immune cell function suggests particular caution for those with dysregulated immune systems. When immune modulation is already problematic, avoiding additives documented to affect immune cell behavior becomes priority.
Post-antibiotic recovery: After antibiotic treatment depletes beneficial bacteria, maximizing probiotic colonization without interference is critical. Any factor that reduces bacterial viability or creates a hostile gut environment—like maltodextrin breaking down protective mucus—can delay or prevent microbiome restoration.
Neurological or cognitive concerns: Given documented effects of silica nanoparticles on the microbiota-gut-brain axis[6], those addressing brain fog, mood disorders, or cognitive function through gut health interventions should avoid additives with demonstrated neurological impacts.
Children and developing microbiomes: Early-life microbiome establishment influences lifelong health trajectories. Conservative approaches to additives with documented biological activity become prudent when supporting developing digestive and immune systems.
Long-term supplementation: Cumulative exposure over months or years increases the potential for subtle effects that may not appear in short-term studies. When planning extended probiotic regimens, minimizing unnecessary additive exposure reduces long-term risk.
Cost-Benefit Reality: Why "Cheaper" Costs You More
Budget-conscious consumers often gravitate toward conventional probiotics with standard fillers because they cost less. But this calculation misses the bigger picture: you're paying for capsules filled with ingredients that don't support your health goals.
Consider the math: if 30% of each capsule contains fillers and flow agents, you're essentially paying for 70% of a supplement. That "bargain" probiotic suddenly isn't such a deal when you realize much of what you're swallowing serves no purpose beyond making manufacturing more profitable for the company.
The real question isn't "Can I afford filler-free probiotics?" but rather "Can I afford to waste money on products that could be significantly better?" When every capsule represents an opportunity to deliver more beneficial bacteria, more prebiotic nourishment, and more gut-supporting compounds, filling that space with manufacturing aids means accepting an inferior product.
Premium manufacturers who eliminate fillers do face higher production costs—specialized equipment, slower speeds, more precise formulation. But those costs deliver tangible value: capsules containing maximum concentrations of ingredients that actually support gut health. You're not paying more for marketing or fancy packaging; you're paying for what's genuinely inside.
MicroBiome Restore: A Case Study in Filler-Free Formulation
Design Philosophy: What Gets Left Out and Why
After reviewing the research on probiotic fillers, examining what's deliberately excluded from formulations like MicroBiome Restore reveals a philosophy: every milligram of space in a capsule should serve the consumer's gut health, not the manufacturer's production efficiency.
No titanium dioxide: Given its association with reduced Lactobacillus populations and inflammatory marker increases[1], this whitening agent has no place in a supplement designed to support the same bacteria it may deplete.
No maltodextrin: Research documenting its mucus-depleting effects and pathogenic biofilm enhancement[2,3] makes it particularly problematic as a probiotic carrier—the delivery mechanism undermines the payload.
No silicon dioxide: While large-particle silica may be relatively safe, the uncertainty around nanoparticle effects[5,6,7] and lack of particle size disclosure on labels makes the precautionary approach to exclude it entirely.
No magnesium stearate: Although safety testing shows it's less dangerous than feared[10], it provides zero benefit to consumers—only manufacturing convenience. Eliminating it removes even theoretical concerns about dissolution delays.
No microcrystalline cellulose: While research shows MCC isn't harmful[8,9], truly optimal formulations prove it's completely unnecessary. That space could hold additional probiotic strains or prebiotic compounds—ingredients that actively support gut health rather than merely occupying volume. Why settle for "not harmful" when you could have "maximally beneficial"?
What's Included: Comprehensive Microbiome Support
The space freed up by eliminating fillers allows for more of what actually matters:
| MicroBiome Restore: Formula Breakdown | |
|---|---|
| 26 Diverse Probiotic Strains | Includes multiple Lactobacillus species, Bifidobacterium strains, and soil-based organisms for comprehensive support across all gut regions |
| 9 Organic Prebiotics | Features acacia senegal gum, inulin, and additional prebiotic fibers that selectively nourish beneficial bacteria without feeding pathogens |
| Fermented Botanical Capsules | Plant-based capsule technology that protects bacteria through stomach acid without requiring animal-derived gelatin |
| Shelf-Stable Formula | Advanced stabilization technology maintains bacterial viability without refrigeration, combining purity with convenience |
| Guaranteed Potency | CFU counts guaranteed through expiration date, not just at manufacture—ensuring viable bacteria when actually consumed |
The Manufacturing Challenge of Filler-Free Production
Producing supplements without flow agents and bulking fillers isn't merely a matter of leaving ingredients out—it requires different equipment, slower production speeds, and more careful formulation. This is why filler-free supplements often cost more: manufacturers accept reduced efficiency to deliver purer products.
Specialized low-heat encapsulation equipment prevents probiotic die-off during production. Precise capsule sizing eliminates the need for bulking agents to fill space. Advanced moisture control systems maintain bacterial stability without silicon dioxide as an anti-caking agent. These investments in production capability enable filler-free formulation while maintaining the quality standards consumers deserve.
Practical Guide: Choosing and Using Probiotic Supplements
Essential Selection Criteria Beyond Fillers
While avoiding problematic fillers is important, it's only one factor in probiotic selection. A filler-free supplement with inadequate CFUs or poorly researched strains still won't deliver results. Comprehensive evaluation requires examining multiple quality indicators:
1. Strain Diversity and Research Support
Look for products with 10-30 different strains spanning multiple genera (Lactobacillus, Bifidobacterium, and ideally some soil-based organisms). Each strain performs different functions—some excel at immune support, others at barrier function, still others at neurotransmitter production. Diversity mirrors the complexity of healthy natural microbiomes and provides insurance against individual variation in strain colonization.
2. Appropriate CFU Count for Your Needs
Colony-forming unit recommendations vary by purpose. Maintenance dosing typically ranges from 1-10 billion CFUs daily. Post-antibiotic recovery or addressing specific conditions may warrant 25-100 billion CFUs. Children generally need lower doses (1-5 billion CFUs). Most importantly, verify guarantees apply through expiration date.
3. Prebiotic Inclusion for Synbiotic Benefits
Research consistently shows that probiotics work better when provided with prebiotic nourishment. Studies demonstrate prebiotics help promote greater diversity of microbes in your gut ecosystem[13] and support successful colonization rather than mere transit through the digestive tract.
4. Storage and Stability Requirements
Traditional thinking held that probiotics must be refrigerated—but modern stabilization technologies enable shelf-stable formulations with equivalent or superior potency. Consider your lifestyle: will you remember to refrigerate consistently? Travel frequently? Shelf-stable options remove friction from adherence.
5. Manufacturing Quality and Testing
Choose products from manufacturers with GMP certification and ideally third-party testing (USP, NSF, or ConsumerLab verification). Check for batch-specific Certificates of Analysis showing actual testing results for potency, purity, and absence of contaminants.
Optimal Timing and Usage Strategies
With or Without Food?
This depends on formulation specifics. Some probiotics include protective technologies allowing empty-stomach consumption for maximum absorption. Others survive better when buffered by food. Follow manufacturer recommendations and maintain consistency—your gut bacteria thrive on routine.
Morning vs. Evening Dosing
Research suggests timing matters less than consistency, though some practitioners recommend evening dosing to align with natural gut repair cycles during sleep. The most important factor: taking them regularly at the same time daily to maintain stable bacterial populations.
Combination with Other Supplements
Probiotics generally combine well with most supplements. However, avoid taking them simultaneously with antimicrobial herbs (oregano oil, berberine) or immediately after hot beverages that might raise internal temperature enough to affect viability.
Supporting Probiotics Through Diet and Lifestyle
Supplements work best as part of comprehensive microbiome support. Dietary strategies amplify probiotic benefits:
Fermented Foods Increase Diversity
A landmark Stanford study found that eating fermented foods like yogurt, kefir, kimchi, and kombucha led to increases in overall microbial diversity, with stronger effects from larger servings[13]. These foods provide different bacterial strains than supplements, creating complementary support.
Prebiotic-Rich Foods Feed Beneficial Bacteria
Include Jerusalem artichoke, chicory root, garlic, onions, leeks, asparagus, bananas (especially slightly green), oats, apples, and flaxseeds regularly. These foods provide the fiber substrates that probiotics ferment into beneficial short-chain fatty acids.
Diverse Plant Foods Build Resilient Microbiomes
Research indicates that fiber diversity matters more than total fiber quantity[13]. Rather than eating massive amounts of the same vegetables, aim for 30+ different plant foods weekly spanning vegetables, fruits, whole grains, legumes, nuts, and seeds.
Lifestyle Factors That Support (or Sabotage) Gut Health
Sleep deprivation, chronic stress, excessive alcohol, and certain medications (particularly antibiotics and NSAIDs) can undermine probiotic efforts. While supplements help, addressing these factors creates conditions where beneficial bacteria can truly thrive.
The Gut-Brain Axis: Why Filler Purity May Affect More Than Digestion
Understanding the Bidirectional Communication Network
One of the most compelling reasons to avoid fillers that disrupt gut microbiota involves effects that extend far beyond digestion. The gut-brain axis represents a bidirectional communication network linking the enteric and central nervous systems through anatomical, endocrine, humoral, metabolic, and immune pathways[14].
This connection allows the brain to influence intestinal activities—but equally important, it allows the gut to influence mood, cognition, and mental health. Research has linked dysbiosis and gut inflammation to multiple mental health conditions including anxiety and depression, with studies showing probiotics can mitigate symptoms comparably to some conventional medications[14].
The neurologic pathway includes the vagus nerve, enteric nervous system, and neurotransmitter activity within the GI tract. Remarkably, the gut produces approximately 90% of the body's serotonin—a critical neurotransmitter affecting mood, sleep, and appetite. Short-chain fatty acids like butyrate and propionate, produced through microbial fermentation, not only maintain gut integrity but also influence brain functions such as mood regulation and cognitive processes[14].
Why Fillers That Affect Microbiota May Impact Mental Health
This gut-brain connection explains why research showing silicon dioxide nanoparticles disrupt the microbiota-gut-brain axis[6] has implications beyond digestive comfort. When additives alter gut bacterial populations, they potentially affect the production of neurotransmitters and neuroactive compounds that influence brain function.
Similarly, maltodextrin's documented effects on mucus layer integrity and inflammatory marker elevation[2] suggest it could indirectly affect mental health through gut-brain signaling. The inflammation it promotes in the gut may translate to inflammatory signals reaching the brain—a mechanism increasingly recognized in depression pathophysiology.
For individuals addressing brain fog, mood disorders, anxiety, or cognitive function through gut health interventions, choosing supplements free from additives with demonstrated neurological impacts becomes particularly important. The filler question transcends digestion—it touches on mental clarity, emotional stability, and cognitive performance.
The Future of Probiotic Supplementation
Emerging Research and Next-Generation Formulations
The field of microbiome research continues evolving rapidly. Current areas of investigation include:
Personalized probiotics based on individual microbiome profiles: Advances in microbiome testing may eventually enable recommendations tailored to each person's unique bacterial composition, targeting specific deficiencies rather than providing generic strain combinations.
Next-generation probiotics from novel bacterial species: Researchers are identifying beneficial bacteria beyond traditional Lactobacillus and Bifidobacterium, including species like Akkermansia muciniphila and Faecalibacterium prausnitzii that show promise for metabolic and inflammatory conditions.
Postbiotics—beneficial compounds produced by probiotics: Some research suggests the metabolites produced by beneficial bacteria may provide benefits even without viable organisms, potentially sidestepping challenges around bacterial survival during storage and digestion.
Precision targeting through strain selection: As research identifies which specific strains address particular health conditions, formulations may become more targeted—using fewer strains chosen specifically for individual health goals rather than broad-spectrum approaches.
Long-term effects of food additives: Ongoing research continues examining how common additives affect microbiome stability and composition over extended periods, likely revealing additional concerns about ingredients currently considered safe.
Individual Variation in Probiotic Response
One consistent research finding is that probiotic effects vary significantly among individuals[15]. This variation explains why some people experience dramatic improvements while others notice minimal changes—and why conclusions about "all probiotics" based on any single study should be viewed cautiously.
Factors influencing individual response include baseline microbiome composition, diet quality and diversity, medication use, stress levels, sleep patterns, exercise habits, and genetic factors affecting gut barrier function and immune response. This complexity means that for some individuals, removing potentially interfering fillers may be more critical than for others whose robust microbiomes resist disruption.
Conclusion: Why Settle for "Good Enough" When Optimal Is Possible?
The research on probiotic fillers reveals an industry pattern: manufacturers choosing production convenience over consumer benefit. While some fillers like titanium dioxide and maltodextrin pose documented risks[1,2,3,4], even the "safe" ones like magnesium stearate and microcrystalline cellulose represent wasted opportunities.
The evidence against harmful fillers is clear:
- Titanium dioxide alters gut microbiota composition, reduces beneficial Lactobacillus, and increases inflammatory markers[1]
- Maltodextrin breaks down protective intestinal mucus, enhances pathogenic biofilm formation, and correlates with IBD incidence[2,3,4]
- Nanoparticle silica exacerbates intestinal inflammation, reduces microvilli counts, and disrupts the microbiota-gut-brain axis[5,6]
- European regulatory agencies have banned titanium dioxide and expressed serious concern about silicon dioxide nanoparticles[7]
But even for the "safe" fillers, the fundamental question remains: Why should consumers accept products where capsule space is wasted on manufacturing convenience rather than maximized for health benefits?
Magnesium stearate may not be toxic[10], but it serves zero purpose for your gut health. Microcrystalline cellulose might even have mild benefits at high doses[8], but supplement manufacturers use it in amounts too small to matter—it's just cheap filler taking up space that could hold more probiotics, more prebiotics, more compounds that actually work.
The supplement industry has trained consumers to compare products based on marginal differences: "Our probiotic has 3 more strains than theirs!" or "We use slightly less magnesium stearate!" This race-to-the-middle creates a market where products compete on being incrementally less bad rather than genuinely optimal.
Products like MicroBiome Restore demonstrate that another approach exists. The formulation combines 26 diverse probiotic strains with 9 organic prebiotics in a shelf-stable formula completely free from titanium dioxide, maltodextrin, silicon dioxide, magnesium stearate, and microcrystalline cellulose. This isn't about marketing "cleaner than competitors"—it's about dedicating every milligram of capsule space to ingredients that support your microbiome.
The manufacturing challenges are real: producing supplements without flow agents requires specialized equipment, accepts slower production speeds, and demands more precise engineering. But those challenges exist to solve the manufacturer's problems, not yours. When companies choose to overcome those challenges rather than pass their manufacturing conveniences to you as fillers, they create products that reach their full potential.
Whether you're recovering from antibiotic treatment, managing digestive discomfort, addressing the gut-brain axis for mental clarity, or optimizing overall wellness, the question isn't just what's in your probiotic—it's what's taking up space that could be something better.
In a market where regulatory oversight remains limited and manufacturers face minimal pre-market requirements, consumer demand for truly optimized formulations drives the only meaningful change. Every purchase of a filler-free supplement sends a message: we refuse to accept products designed for manufacturing efficiency instead of maximum efficacy.
Ready to experience what probiotics can accomplish when formulation prioritizes your health over production convenience? Explore MicroBiome Restore and discover the difference between products that compete on being "less bad" versus products engineered to be genuinely optimal.
References
- Rinninella, E., Raoul, P., Cintoni, M., Franceschi, F., Miggiano, G.A.D., Gasbarrini, A., & Mele, M.C. (2021). Impact of Food Additive Titanium Dioxide on Gut Microbiota Composition, Microbiota-Associated Functions, and Gut Barrier: A Systematic Review of In Vivo Animal Studies. International Journal of Environmental Research and Public Health, 18(4), 2008. https://pmc.ncbi.nlm.nih.gov/articles/PMC7922260/
- Laudisi, F., Di Fusco, D., Dinallo, V., Stolfi, C., Di Grazia, A., Marafini, I., et al. (2019). The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cellular and Molecular Gastroenterology and Hepatology, 7(2), 457-473. https://www.sciencedirect.com/science/article/pii/S2352345X18301218
- Nickerson, K.P., & McDonald, C. (2015). Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes, 6(1), 78-83. https://pmc.ncbi.nlm.nih.gov/articles/PMC4615306/
- Almutairi, R., Basson, A.R., Wearsh, P., Cominelli, F., & Rodriguez-Palacios, A. (2022). Validity of food additive maltodextrin as placebo and effects on human gut physiology: systematic review of placebo-controlled clinical trials. European Journal of Nutrition, 61(6), 2853-2871. https://pmc.ncbi.nlm.nih.gov/articles/PMC9835112/
- Yan, J., Wang, D., Li, K., Chen, Q., Lai, W., Tian, L., et al. (2020). Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environment International, 145, 106123. https://pubmed.ncbi.nlm.nih.gov/32977129/
- Diao, J., Xia, Y., Jiang, X., Huang, J., & Zhu, N. (2021). Silicon dioxide nanoparticles induced neurobehavioral impairments by disrupting microbiota-gut-brain axis. Journal of Nanobiotechnology, 19, 174. https://pubmed.ncbi.nlm.nih.gov/34112173/
- EFSA Panel on Food Additives and Nutrient Sources. (2018). Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA Journal, 16(1), e05088. https://pmc.ncbi.nlm.nih.gov/articles/PMC7009582/
- Nguyen, N.K., Deehan, E.C., Zhang, Z., Jin, M., Baskota, N., Perez-Muñoz, M.E., et al. (2022). Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome, 10, 77. https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-022-01248-5
- Zheng, M., Han, R., Yuan, Y., Xue, Y., Yue, T., Liu, M., et al. (2022). Structural Characteristics of Inulin and Microcrystalline Cellulose and Their Effect on Ameliorating Colitis and Altering Colonic Microbiota in Dextran Sodium Sulfate-Induced Colitic Mice. Journal of Agricultural and Food Chemistry, 70(12), 3688-3703. https://pmc.ncbi.nlm.nih.gov/articles/PMC8991927/
- Ramalingam, P., Yoo, S.W., & Ko, Y.T. (2017). Magnesium stearate, a widely-used food additive, exhibits a lack of in vitro and in vivo genotoxic potential. Toxicology Reports, 4, 554-559. https://pmc.ncbi.nlm.nih.gov/articles/PMC5655391/
- Sanchez-Alcoholado, L., Ordóñez, R., Otero, A., Plaza-Andrades, I., Laborda-Illanes, A., Medina, J.A., et al. (2020). Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients, 12(9), 2889. https://pubmed.ncbi.nlm.nih.gov/32971775/
- da Conceição, R.C.N., Batista, R.D., Zimmer, F.M.d.A.L., Trindade, I.K.M., de Almeida, A.F., & Santos, C.C.A.d.A. (2021). Effect of co-encapsulation using a calcium alginate matrix and fructooligosaccharides with gelatin coating on the survival of Lactobacillus paracasei cells. LWT - Food Science and Technology, 151, 112179. https://pubmed.ncbi.nlm.nih.gov/34531028/
- Wastyk, H.C., Fragiadakis, G.K., Perelman, D., Dahan, D., Merrill, B.D., Yu, F.B., et al. (2021). Gut-microbiota-targeted diets modulate human immune status. Cell, 184(16), 4137-4153. https://pubmed.ncbi.nlm.nih.gov/34256014/
- Clapp, M., Aurora, N., Herrera, L., Bhatia, M., Wilen, E., & Wakefield, S. (2017). Gut microbiota's effect on mental health: The gut-brain axis. Clinics and Practice, 7(4), 987. https://pmc.ncbi.nlm.nih.gov/articles/PMC5641835/
- Zmora, N., Zilberman-Schapira, G., Suez, J., Mor, U., Dori-Bachash, M., Bashiardes, S., et al. (2018). Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell, 174(6), 1388-1405. https://pubmed.ncbi.nlm.nih.gov/30193112/











Share and get 15% off!
Simply share this product on one of the following social networks and you will unlock 15% off!