Top Benefits of Combining Prebiotics and Probiotics
Discover how synbiotic formulations enhance gut health, support immune function, and promote metabolic wellness through the powerful synergy of prebiotics and probiotics
Your gut microbiome—the complex ecosystem of trillions of microorganisms residing in your digestive tract—plays a fundamental role in virtually every aspect of your health. From digesting food and producing essential vitamins to regulating immune responses and influencing mental wellbeing, these microscopic inhabitants work tirelessly to keep your body functioning optimally. Yet modern lifestyles characterized by processed foods, chronic stress, antibiotic use, and environmental toxins continually challenge this delicate microbial balance.
Enter prebiotics and probiotics: two complementary approaches to supporting gut health that have garnered substantial scientific attention. Probiotics introduce beneficial living microorganisms to your digestive system, while prebiotics provide the specialized nutrition these bacteria need to thrive. When combined—creating what scientists call synbiotics—these components produce effects that exceed what either can achieve independently.[1]
Recent research published in 2024 and 2025 reveals increasingly sophisticated insights into how prebiotic-probiotic combinations modulate gut microbiota, enhance metabolic function, strengthen immune responses, and support mental health through the gut-brain axis.[2] Multi-strain formulations containing both Lactobacillus and Bifidobacterium species alongside diverse prebiotic fibers consistently demonstrate superior clinical outcomes compared to single-strain products.[3]
This comprehensive guide examines the science-backed benefits of combining prebiotics and probiotics, explores the mechanisms through which synbiotics enhance health, and explains why formulations like MicroBiome Restore™—featuring 26 probiotic strains, 9 certified organic prebiotics, and innovative pullulan capsule technology—represent the evolution of gut health supplementation.
Key Takeaways
- Synbiotics outperform individual components: Research demonstrates that combining prebiotics with probiotics creates synergistic effects that amplify benefits for digestive health, immune function, and metabolic regulation beyond what either achieves alone.[1]
- Short-chain fatty acids drive core benefits: When probiotics ferment prebiotic fibers, they produce acetate, propionate, and butyrate—metabolites that nourish gut cells, reduce inflammation, strengthen barrier function, and improve insulin sensitivity.[4] The primary way prebiotics benefit gut health is through fermentation into short-chain fatty acids—our guide on how to increase butyrate and SCFAs covers this process in detail.
- Multi-strain formulations demonstrate superior efficacy: Clinical trials consistently show that products containing multiple Lactobacillus and Bifidobacterium species produce better outcomes than single-strain alternatives for conditions ranging from IBS to metabolic syndrome.[3]
- Gut barrier integrity underpins systemic health: The combination strengthens tight junction proteins between intestinal cells, preventing "leaky gut" that contributes to inflammation, autoimmune conditions, and metabolic disorders.[5]
- Metabolic benefits extend beyond digestion: Synbiotics improve insulin sensitivity, support healthy weight management, reduce inflammatory markers, and modulate lipid profiles in individuals with obesity and type 2 diabetes.[6]
- The gut-brain axis connects digestion and mental health: Probiotics produce neurotransmitters and reduce inflammatory signaling that affects mood, anxiety, and cognitive function—benefits amplified when combined with prebiotics.[7]
- Capsule technology matters for efficacy: Advanced delivery systems like pullulan capsules provide delayed release to the intestines, oxygen barrier protection, and prebiotic properties that standard HPMC or gelatin capsules cannot match.[8]
Understanding the Synbiotic Advantage: When Prebiotics and Probiotics Unite
The concept of synbiotics emerged from a simple yet profound observation: probiotics work significantly better when provided with their preferred fuel. A 2025 systematic review published in Nutrition Journal analyzing randomized controlled trials across diverse populations found that synbiotic supplementation notably increased beneficial bacteria abundance, with prebiotics showing a standardized mean difference of 1.09 for Bifidobacterium and probiotics showing 0.40—yet synbiotic combinations demonstrated even greater shifts in microbial composition when specific strains were paired with targeted fibers.[9]

Prebiotics are specialized plant fibers—including inulin, fructooligosaccharides, galactooligosaccharides, and resistant starch—that resist digestion in the upper gastrointestinal tract and arrive intact in the colon. There, specific bacterial species possessing the enzymatic machinery to break down these complex carbohydrates ferment them into beneficial metabolites. This selective nourishment promotes the growth of health-promoting bacteria like Lactobacillus and Bifidobacterium species while creating conditions that discourage pathogenic microbes.[10]
Probiotics, meanwhile, are live microorganisms that confer health benefits when administered in adequate amounts. The most extensively researched probiotic families include Lactobacillus species (now reclassified into multiple genera including Lactiplantibacillus and Lacticaseibacillus), Bifidobacterium species, and spore-forming Bacillus species. Each strain possesses unique properties—some excel at producing specific vitamins, others at strengthening gut barrier function, and still others at modulating immune responses.[11]
The Mechanisms of Synbiotic Synergy
When prebiotics and probiotics work together, several complementary mechanisms amplify their individual effects:
Enhanced colonization and survival: Prebiotic fibers provide immediate nourishment for probiotic bacteria upon arrival in the intestines, increasing their ability to establish colonies and survive in the competitive gut environment. A 2025 study published in Bioscience of Microbiota, Food and Health demonstrated that Bifidobacterium animalis subsp. lactis combined with inulin showed significantly increased acetate, propionate, and butyrate production compared to probiotics alone.[12]
Selective microbial modulation: Different prebiotic fibers preferentially feed specific bacterial families. For instance, inulin primarily nourishes Bifidobacterium, while resistant starch supports certain Ruminococcus and butyrate-producing Clostridium clusters. This selectivity allows for targeted microbiome modification.[13]
Metabolic cross-feeding networks: Some bacteria ferment prebiotics into intermediates that other species then convert into more specialized metabolites. For example, Bifidobacterium may produce lactate that butyrate-producing bacteria like Faecalibacterium prausnitzii or Eubacterium hallii then convert into butyrate—creating cooperative metabolic relationships that benefit overall gut ecology.[14]

MicroBiome Restore™: Synbiotic Science in Action
MicroBiome Restore™ exemplifies the synbiotic approach by combining 26 diverse probiotic strains—including multiple Lactobacillus, Bifidobacterium, and soil-based Bacillus species—with 9 certified organic prebiotics. This comprehensive formula includes maitake mushroom, fig fruit, Jerusalem artichoke, acacia senegal gum, Norwegian kelp, bladderwrack, and oarweed—each selected to nourish different bacterial populations and provide diverse health-promoting compounds.
Unlike formulations containing microcrystalline cellulose or magnesium stearate, MicroBiome Restore contains zero harmful fillers. The formula is encapsulated in fermented pullulan capsules that provide delayed release, superior oxygen barrier properties, and prebiotic benefits—advantages that standard HPMC or hypromellose capsules cannot offer.[8]
Short-Chain Fatty Acids: The Metabolic Currency of Gut Health
Perhaps the most critical benefit of combining prebiotics and probiotics lies in their ability to dramatically increase production of short-chain fatty acids (SCFAs)—particularly acetate, propionate, and butyrate. These small molecules, produced when gut bacteria ferment dietary fibers and prebiotics, serve as metabolic signaling compounds with far-reaching effects throughout the body.[4]
A groundbreaking 2025 randomized controlled trial published in Frontiers in Cellular and Infection Microbiology examined the effects of short-chain fatty acid-producing probiotic metabolites on irritable bowel syndrome patients. After 12 weeks of intervention, researchers observed significantly increased levels of acetate, propionate, and butyrate (all P<0.01), accompanied by substantial improvements in intestinal permeability markers including occludin and claudin-1—tight junction proteins essential for gut barrier integrity.[15]

Butyrate: The Gut Cell's Preferred Fuel
Butyrate provides approximately 70% of the energy required by colonocytes—the epithelial cells lining the colon. This relationship is so critical that butyrate deficiency has been linked to inflammatory bowel diseases, colon cancer risk, and compromised barrier function. Beyond serving as fuel, butyrate exhibits potent anti-inflammatory properties by inhibiting histone deacetylase (HDAC) enzymes, thereby modulating gene expression in ways that reduce inflammatory cytokine production.[16]
Research published in eBioMedicine by The Lancet emphasizes that butyrate-producing bacteria like Faecalibacterium prausnitzii represent key targets for therapeutic intervention in inflammatory bowel disease. The review notes that prebiotic or probiotic treatments specifically designed to support these butyrate producers show particular promise for IBD management.[17]
Propionate and Acetate: Systemic Metabolic Regulators
While butyrate primarily acts locally in the colon, propionate and acetate enter systemic circulation and influence distant organs. Propionate activates G-protein-coupled receptors on adipocytes and pancreatic beta cells, improving insulin sensitivity and glucose homeostasis. Acetate serves as a substrate for cholesterol synthesis in the liver and acts as a signaling molecule that can reduce appetite through effects on the central nervous system.[18]
A 2025 systematic review examining the effects of probiotics, prebiotics, and synbiotics on type 2 diabetes found that these interventions efficiently decreased endotoxin and lipopolysaccharide levels while promoting SCFA production—effects that correlated with improved glucose homeostasis and reduced insulin resistance.[19]
SCFA Production: Synbiotics vs. Probiotics Alone
Probiotics Only: Moderate SCFA increases, highly dependent on existing gut microbiota composition and dietary fiber intake
Prebiotics Only: Feeds existing bacteria, but effects limited if beneficial species are depleted
Synbiotic Combination: Introduces beneficial bacteria AND provides their preferred fuel, resulting in significantly greater SCFA production and more consistent clinical benefits across individuals with varying baseline microbiomes[12]
The impact of SCFAs extends to immune regulation, with all three major SCFAs influencing T-cell differentiation, enhancing regulatory T-cell function, and reducing pro-inflammatory cytokines like TNF-α and IL-6 while promoting anti-inflammatory IL-10 production.[20]
Strengthening the Gut Barrier: Protection Against Systemic Inflammation
The intestinal epithelium represents your body's largest interface with the external environment, covering approximately 400 square meters and encountering a constant stream of potentially harmful substances alongside nutrients. This single-cell-layer barrier must simultaneously allow selective absorption of essential compounds while preventing passage of toxins, pathogens, and incompletely digested food particles. When this barrier becomes compromised—a condition often called "leaky gut" or increased intestinal permeability—systemic inflammation, immune dysregulation, and metabolic dysfunction can result.[5]

Tight Junction Proteins: The Gatekeepers
Tight junctions are protein complexes—including occludin, claudins, and zonula occludens (ZO) proteins—that seal the spaces between intestinal epithelial cells, controlling paracellular permeability. Recent research demonstrates that specific probiotic strains directly upregulate these protective proteins. A 2024 study found that Lactobacillus rhamnosus GG increased occludin and claudin-1 expression, while Bifidobacterium infantis enhanced ZO-1 levels, thereby fortifying the epithelial barrier.[21]
The prebiotic component contributes through SCFA production. Butyrate, in particular, strengthens tight junctions by activating AMP-activated protein kinase (AMPK) pathways that promote tight junction assembly and by reducing oxidative stress that can damage these proteins. The combined effect of probiotics directly supporting tight junction expression and prebiotics providing the butyrate that maintains them creates robust barrier protection.[22]
Mucus Layer Enhancement
The intestinal mucus layer provides an additional protective barrier, preventing direct bacterial contact with epithelial cells while allowing nutrient absorption. Research shows that butyrate stimulates expression of mucin genes, increasing production of the protective mucus glycoproteins that coat the intestinal surface. Certain probiotic strains, particularly Lactobacillus plantarum and Bifidobacterium species, directly enhance mucus production and modify mucus composition to be more resistant to degradation by pathogenic bacteria.[23]
Reducing Metabolic Endotoxemia
When barrier function is compromised, lipopolysaccharide (LPS) from gram-negative bacteria can translocate into circulation, triggering "metabolic endotoxemia"—a state of chronic low-grade inflammation linked to insulin resistance, obesity, and cardiovascular disease. A 2025 systematic review documented that probiotic, prebiotic, and synbiotic administration efficiently decreased circulating LPS and endotoxin levels in type 2 diabetes patients, with corresponding improvements in inflammatory markers and glucose homeostasis.[19]
By strengthening tight junctions, enhancing mucus production, and promoting anti-inflammatory SCFA production, the prebiotic-probiotic combination addresses multiple aspects of barrier integrity simultaneously. This comprehensive approach explains why synbiotics show particular promise for conditions characterized by increased intestinal permeability, including inflammatory bowel disease, irritable bowel syndrome, allergies, and metabolic syndrome.
Metabolic Health Benefits: Supporting Healthy Weight and Insulin Sensitivity
The relationship between gut microbiota and metabolic health has emerged as one of the most compelling areas of microbiome research. Individuals with obesity, metabolic syndrome, and type 2 diabetes consistently exhibit distinct microbial signatures characterized by reduced diversity, decreased abundance of beneficial species like Faecalibacterium prausnitzii and Akkermansia muciniphila, and increased ratios of Firmicutes to Bacteroidetes phyla.[6]

Insulin Sensitivity and Glucose Homeostasis
A comprehensive review published in American Journal of Physiology-Endocrinology and Metabolism examined 38 randomized clinical trials encompassing 2,086 patients with diabetes. The analysis revealed significant benefits from prebiotics, probiotics, and synbiotics, including reduced HbA1c levels (−2.17 mmol/mol), decreased fasting plasma glucose (−0.58 mmol/L, P<0.01), and reduced insulinemia (−10.51 pmol/L, P<0.01).[24]
The mechanisms underlying these improvements are multifaceted. SCFAs activate G-protein-coupled receptors (GPR41/43) on enteroendocrine cells, promoting secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY)—hormones that enhance insulin secretion and improve insulin sensitivity. Additionally, by reducing systemic inflammation and LPS-induced metabolic endotoxemia, synbiotics address one of the primary drivers of insulin resistance.[25]
A 2025 randomized, double-blind, placebo-controlled trial published in Clinical Nutrition found that synbiotic supplementation (combining multiple probiotic strains with prebiotic fibers) surpassed probiotics alone in improving type 2 diabetes mellitus outcomes. The synbiotic group showed greater reductions in fasting glucose, HbA1c, and inflammatory markers compared to probiotic-only supplementation.[26]
Weight Management and Body Composition
Multiple systematic reviews with meta-analyses have demonstrated favorable effects of prebiotics, probiotics, and particularly synbiotics on obesity indicators including weight, BMI, and waist circumference. The anti-obesity effects appear more pronounced in individuals who are already overweight or obese compared to those of normal weight.[27]
A 2025 multicentric clinical trial published in Cureus evaluated a probiotic-fiber blend formulation in obese adults. The study found that the synbiotic group experienced significant reductions in body weight, BMI, waist circumference, and metabolic markers compared to placebo. The formulation contained Bifidobacterium and Lactobacillus species along with prebiotic fibers (FOS, inulin, glucomannan), which synergistically enhanced SCFA production and promoted insulin sensitivity.[28]
The mechanisms include:
- Appetite regulation: SCFAs stimulate satiety hormone release (GLP-1, PYY) while reducing hunger hormone ghrelin
- Energy harvest modulation: Certain beneficial bacteria extract fewer calories from food than opportunistic species
- Fat storage regulation: Gut bacteria influence expression of genes involved in fat metabolism and storage
- Inflammation reduction: Chronic low-grade inflammation associated with obesity decreases when gut barrier integrity improves[29]
Lipid Profile Improvements
Beyond glucose metabolism, synbiotics demonstrate beneficial effects on lipid parameters. The meta-analysis referenced earlier found significant reductions in total cholesterol (−0.14 mmol/L, P=0.02) and triglycerides (−0.11 mmol/L, P=0.01) among diabetes patients receiving prebiotic, probiotic, or synbiotic interventions.[24]
A 2025 study examining Lactobacillus plantarum and Bifidobacterium longum found both strains exhibited anti-lipidemic properties in vitro, with lipase inhibition activities of 54% and 68% respectively—suggesting mechanisms by which these probiotics may reduce dietary fat absorption and improve lipid profiles.[30]
Immune System Modulation: Balancing Protection and Tolerance
Approximately 70-80% of your immune system resides in gut-associated lymphoid tissue (GALT), making the intestinal immune interface critically important for overall immune function. The gut must maintain a delicate balance—mounting robust responses against pathogens while tolerating beneficial commensal bacteria and harmless food antigens. This immunological tightrope walk depends heavily on signals from the gut microbiota, positioning synbiotics as powerful immune modulators.[31]
Regulatory T Cell Enhancement
Probiotics promote development and function of regulatory T cells (Tregs)—specialized immune cells that prevent excessive inflammatory responses and maintain tolerance to harmless antigens. Specific strains including Lactobacillus plantarum, Lactobacillus rhamnosus, and Bifidobacterium longum have demonstrated the ability to induce Treg expansion and enhance their suppressive function.[32]
A 2024 comprehensive review published in Nutrients documented that probiotics immediately activate anti-inflammatory mechanisms by producing anti-inflammatory cytokines such as IL-4, IL-10, IL-11, and IL-13 while inhibiting pro-inflammatory cytokines including IL-1, IL-6, and TNF-α through pathways involving Tregs and T helper cells.[33]
Innate Immunity Enhancement
Beyond adaptive immunity, synbiotics enhance innate immune defenses. Research shows that certain probiotic strains increase natural killer (NK) cell activity and macrophage phagocytic capacity. A 2023 study found that Lactobacillus rhamnosus and Bifidobacterium longum suppressed LPS-induced natural killer cell cytotoxicity and macrophage phagocytosis dysfunction, helping normalize immune responses that had been disrupted by inflammatory stimuli.[34]
Antimicrobial Compound Production
Many probiotic strains produce antimicrobial substances that directly inhibit pathogen growth, including:
- Organic acids: Lactic acid and acetic acid lower local pH, creating inhospitable conditions for many pathogens
- Bacteriocins: Protein compounds that disrupt pathogen cell membranes
- Hydrogen peroxide: Produced by certain Lactobacillus species, particularly in oxygen-rich environments
- Biosurfactants: Compounds that prevent pathogen adhesion to intestinal surfaces[35]
Allergy and Autoimmunity Applications
The immune-modulating effects of synbiotics show particular promise for allergic conditions. A 2024 randomized controlled trial published in the Journal of Korean Medical Science found that a combination of Bifidobacterium longum and Lactobacillus plantarum significantly reduced allergic nasal symptoms in children with perennial allergic rhinitis. The treatment reduced total nasal symptom scores and improved quality of life, accompanied by decreases in Th2 cytokines in nasal tissues.[36]
Multiple studies have also documented benefits for inflammatory bowel diseases. A 2025 review in Frontiers in Systems Biology concluded that certain probiotic formulations—particularly mixed-strain combinations of Lactobacillus and Bifidobacterium species alongside prebiotic compounds like fructooligosaccharides—proved effective in improving clinical, immunological, and symptomatic aspects of IBD.[37]
The Gut-Brain Axis: Mental Wellness Through Microbial Balance
The discovery that gut bacteria communicate bidirectionally with the central nervous system through neural, endocrine, and immune pathways has revolutionized our understanding of mental health. This "gut-brain axis" helps explain why digestive complaints so frequently accompany mood disorders and why supporting gut health can yield unexpected improvements in anxiety, depression, and cognitive function.[7]

Neurotransmitter Production
Gut bacteria synthesize or influence production of numerous neurotransmitters and neuroactive compounds. Research demonstrates that Lactobacillus acidophilus and Bifidobacterium longum can upregulate serotonin transporter (SERT) expression in intestinal epithelial cells—a mechanism by which these probiotics may influence mood, as serotonin regulates not only emotional state but also intestinal motility and sensation.[38]
Beyond serotonin (approximately 90% of which is produced in the gut), certain strains produce or stimulate production of GABA (the primary inhibitory neurotransmitter), dopamine, and various neuropeptides. Specific probiotic strains including Lactobacillus rhamnosus and Bifidobacterium longum have shown particular efficacy in preclinical studies for their neurological effects.[39]
Inflammation and the Brain
Systemic inflammation originating from gut dysbiosis and increased intestinal permeability can reach the brain, where inflammatory mediators affect neuronal function and neurotransmitter metabolism. By reducing gut inflammation, strengthening barrier function, and decreasing circulating inflammatory cytokines, synbiotics address one pathway linking gut health to mental wellness.
A comprehensive 2024 review noted that probiotics suppress LPS-induced inflammatory responses and decrease production of pro-inflammatory cytokines like TNF-α and IL-6 that can cross the blood-brain barrier and contribute to depression and anxiety.[33]
Vagus Nerve Communication
The vagus nerve—the primary neural pathway connecting gut and brain—transmits signals bidirectionally. Research indicates that certain probiotics can stimulate vagal afferent neurons, sending calming signals to the brain that may reduce anxiety and stress responses. Lactobacillus rhamnosus in particular has been shown to activate vagal pathways that influence brain regions involved in emotional regulation.[40]
Clinical Applications for Mental Health
While research on gut-brain interventions is still evolving, preliminary evidence suggests benefits for various conditions. Studies have documented improvements in anxiety, depression, and cognitive function with specific probiotic strains, though results vary considerably based on the strains used, dosages, and patient populations.
For those interested in leveraging the gut-brain connection, supporting gut health through comprehensive microbiome restoration represents a promising complementary approach to traditional mental health interventions. The multi-strain, prebiotic-inclusive formulation in MicroBiome Restore™ addresses multiple aspects of the gut-brain axis simultaneously.
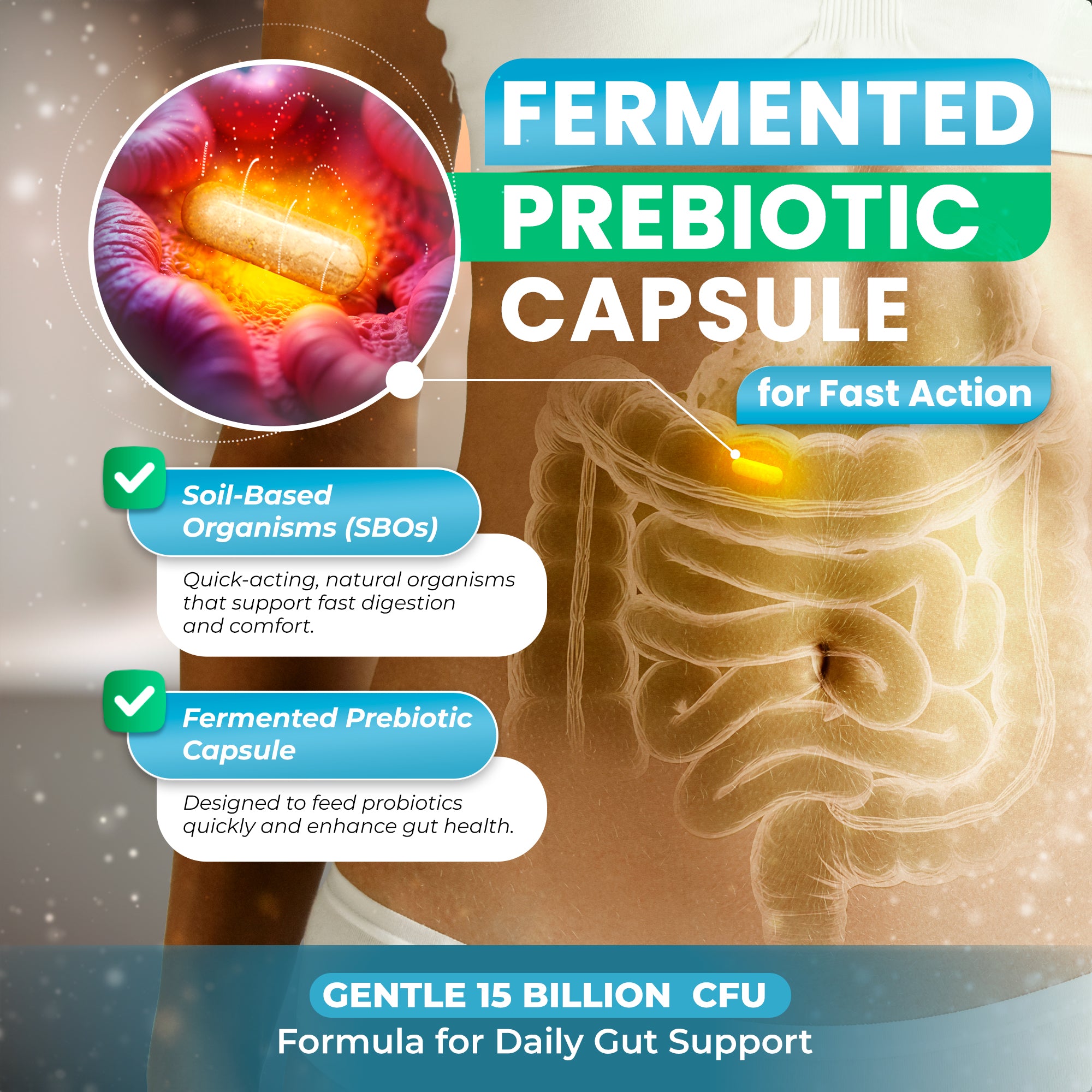
Delivery Matters: Why Pullulan Capsules Represent a Technological Advancement
Even the most carefully formulated probiotic-prebiotic combination will underperform if the delivery system fails to protect bacterial viability or ensure appropriate release timing. Recent research highlights significant differences between capsule materials, with pullulan emerging as a superior option for probiotic delivery compared to standard HPMC (hypromellose) or gelatin capsules.[8]

Superior Oxygen Barrier Properties
Oxygen exposure rapidly degrades probiotic viability, particularly for oxygen-sensitive strains. Pullulan's oxygen barrier properties significantly exceed those of HPMC and gelatin capsules, extending shelf life and maintaining bacterial counts closer to label claims throughout storage. Industry sources report that pullulan's oxygen barrier is substantially stronger than alternatives, helping ensure that consumers receive viable probiotics even months after manufacturing.[41]
Delayed Release for Optimal Delivery
Standard capsules dissolve rapidly in stomach acid, exposing probiotics to harsh conditions that kill many strains before they reach the intestines where colonization occurs. Delayed-release capsules resist dissolution in acidic gastric conditions, protecting their contents until reaching the higher pH environment of the intestines.
Clinical data supports the importance of this feature. Research has shown that delayed-release encapsulation significantly enhances probiotic survival in simulated gastrointestinal models, leading to more consistent ingredient delivery and improved consumer outcomes. Businesses using delayed-release technology report higher customer satisfaction and stronger repurchase rates compared to standard formulations.[42]
Pullulan capsules naturally provide delayed-release properties without requiring enteric coating, as the material resists gastric acid dissolution while breaking down in the more alkaline intestinal environment. A 2023 study examining enteric pullulan hard capsules found they remained stable for 2 hours in acidic conditions (pH 1.2) before rupturing in phosphate buffer simulating the small intestine (pH 6.8).[43]
Prebiotic Properties: More Than Just Protection
Perhaps most remarkably, pullulan itself exhibits prebiotic characteristics. This water-soluble polysaccharide can be metabolized by colonic enzymes and microorganisms into simpler sugars that beneficial bacteria then ferment into SCFAs. Multiple studies have documented pullulan's prebiotic potential, showing it selectively enhances growth of Lactobacillus and Bifidobacterium species.[44]
A 2019 study published in Frontiers in Microbiology demonstrated that pullulan nanoparticles functioned as effective prebiotics, selectively enhancing Lactobacillus plantarum growth and antibacterial activity. A 2017 investigation on human fecal microbiota showed pullulan exhibited selective effects on colonic bacteria, increasing fermentation activity and favorably modifying gut microbiota composition, particularly benefiting Bifidobacterium populations.[8]
This means pullulan capsules do triple duty: protecting probiotics from oxygen degradation, ensuring delayed release to the intestines, and providing prebiotic nourishment once they arrive—a functional synergy that standard capsule materials cannot match.
The MicroBiome Restore™ Advantage
MicroBiome Restore™ utilizes fermented pullulan capsules that provide all these advantages while avoiding the potential concerns associated with HPMC/hypromellose capsules. While HPMC demonstrates adequate stability, it fundamentally lacks the bioactive prebiotic properties that distinguish pullulan as a functional ingredient that actively supports your gut ecosystem rather than merely protecting its contents.
Multi-Strain Formulations: Why Diversity Matters
Your gut microbiome hosts hundreds of bacterial species, each with specialized functions and metabolic capabilities. The concept that a single probiotic strain could address this complexity seems increasingly implausible as research advances. Indeed, clinical trials consistently demonstrate that multi-strain probiotics outperform single-strain formulations across numerous health outcomes.[3]
Complementary Mechanisms and Synergistic Effects
Different bacterial species and strains offer distinct benefits. Lactobacillus acidophilus excels at producing lactic acid that inhibits pathogens, Bifidobacterium longum specializes in vitamin synthesis and immune modulation, Lactobacillus plantarum produces diverse antimicrobial compounds, and Lactobacillus reuteri uniquely synthesizes reuterin with broad antimicrobial activity. When combined, these strains work synergistically—each contributing its specialized functions while potentially supporting the others' activities through metabolic cross-feeding.[45]
A comprehensive 2024 review documenting anti-inflammatory and curative effects of probiotics across major organs noted that multi-strain formulations demonstrate greater efficacy than single-strain administration due to complementary or even synergistic effects.[33]
Colonization Across the Digestive Tract
Different probiotic species preferentially colonize different intestinal regions. Lactobacillus species tend to dominate in the small intestine, while Bifidobacterium species primarily colonize the colon. Spore-forming Bacillus species survive harsh conditions that would kill other probiotics, potentially reaching areas other strains cannot. A multi-strain formula ensures comprehensive coverage across the entire gastrointestinal tract.[46]
Individual Variability and Resilience
Gut microbiome composition varies dramatically between individuals based on genetics, diet, medications, and environmental exposures. A probiotic strain that colonizes effectively in one person may fail to establish in another due to their unique microbial ecosystem. Multi-strain formulations increase the probability that several strains will successfully colonize, providing more consistent benefits across diverse populations.
The 26-Strain Approach
MicroBiome Restore™ contains 26 distinct probiotic strains selected for their complementary functions and clinical research support:
Bifidobacterium Species: Including B. bifidum, B. breve, B. infantis, B. lactis, and B. longum subsp. longum—species critical for immune regulation, vitamin production, and colonization resistance against pathogens
Lactobacillus Species: Including L. acidophilus, L. buchneri, L. casei, L. delbrueckii subsp. bulgaricus, L. fermentum, L. gasseri, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, and L. salivarius—strains with diverse capabilities including lactic acid production, antimicrobial compound synthesis, and immune modulation
Soil-Based Organisms: Including Bacillus coagulans, B. clausii, B. licheniformis, B. pumilus, and B. subtilis—spore-forming species that survive harsh conditions and offer unique benefits through their specialized metabolic capabilities. Learn more about the unique advantages of soil-based probiotics.
Additional Strains: Including Enterococcus faecium, Lactococcus lactis subsp. lactis, Pediococcus acidilactici, P. pentosaceous, and Streptococcus thermophilus—adding further metabolic diversity and functional capabilities
This comprehensive strain profile addresses multiple aspects of gut health simultaneously, from digestive comfort and immune support to metabolic regulation and barrier function—benefits that no single-strain product could provide.
Practical Application: Integrating Synbiotics Into Your Wellness Routine
Understanding the science behind prebiotics and probiotics is valuable, but translating that knowledge into practical strategies yields the actual benefits. Here's how to effectively incorporate synbiotics into your daily routine for maximum impact.
Dosage and Timing Considerations
While CFU (colony-forming unit) counts receive substantial marketing attention, strain diversity and viability often matter more than raw numbers. Research shows effective outcomes with formulations ranging from 5 billion to 50 billion CFU daily, with the optimal dose depending on specific strains and health goals. The World Gastroenterology Organisation notes that effective doses vary dramatically by strain—for instance, Bifidobacterium longum subsp. longum 35624 proves effective at 100 million CFU daily, while other products require 300-450 billion CFU three times daily.[47]
Timing recommendations vary, but many experts suggest taking probiotics on an empty stomach or with a light meal to minimize exposure to harsh stomach acid and digestive enzymes. However, the delayed-release properties of advanced capsule technologies like pullulan reduce the importance of precise timing.
Combining Supplements with Dietary Sources
While synbiotic supplements provide targeted support, dietary sources of probiotics and prebiotics offer complementary benefits. Natural probiotic sources include fermented foods like yogurt, kefir, sauerkraut, kimchi, kombucha, and traditional fermented vegetables. Prebiotic-rich foods include Jerusalem artichoke, chicory root, garlic, onions, leeks, asparagus, bananas (especially slightly underripe), oats, and apples.
The most effective approach combines daily synbiotic supplementation with regular consumption of fermented foods and prebiotic-rich vegetables—creating a comprehensive strategy that introduces beneficial bacteria while continuously nourishing them through dietary fiber.
Managing Initial Adjustment
Some individuals experience temporary digestive changes when beginning probiotic supplementation, including mild gas, bloating, or changes in bowel movements. These effects typically resolve within a few days to two weeks as the gut microbiome adjusts. Starting with a moderate dose and gradually increasing can minimize discomfort for sensitive individuals.
For those prone to bloating with probiotics, formulations emphasizing strains that don't produce significant gas (like certain Lactobacillus gasseri and Bifidobacterium infantis strains) alongside gentle prebiotics like acacia fiber may prove better tolerated than those containing highly fermentable fibers.
Supporting Your Microbiome Holistically
While synbiotics form a cornerstone of microbiome support, other lifestyle factors significantly influence gut health:
- Dietary diversity: Consuming a wide variety of plant foods (aim for 30+ different types weekly) provides diverse fibers that nourish different bacterial species
- Stress management: Chronic stress alters gut motility and permeability; practices like meditation, yoga, or regular exercise support gut health
- Adequate sleep: Sleep deprivation disrupts circadian rhythms that govern gut microbiome composition
- Judicious antibiotic use: When antibiotics are necessary, taking probiotics during and after treatment can help restore microbial balance
- Mineral support: Trace minerals serve as essential cofactors for numerous bacterial enzymes[48]
The Complete Gut Health System
The Gut Essentials Protocol combines MicroBiome Restore™ with our X-Cellerator Full Spectrum Minerals for comprehensive support. The liquid mineral formula provides over 70 trace minerals in ionic form, supplying the essential cofactors that support both bacterial metabolism and your own digestive enzyme function—creating synergy that addresses gut health from multiple angles simultaneously.
Choosing Quality: What to Look for in a Synbiotic Supplement
The explosive growth of the probiotic market has yielded countless products of varying quality. Selecting an effective synbiotic supplement requires evaluating several critical factors beyond marketing claims.
Strain Identification and Research Support
Quality products list specific strain designations (genus, species, and strain), not just generic terms like "probiotic blend." Look for strains with published clinical research documenting their specific health benefits. For example, Lactobacillus rhamnosus GG has extensive research for digestive and immune health, while Bifidobacterium longum 35624 specifically demonstrates benefits for IBS.
CFU Guarantee Through Expiration
Many products guarantee CFU counts only at time of manufacture, meaning bacterial viability may decline substantially before consumption. Quality manufacturers provide CFU guarantees through the expiration date, often building in overages to ensure label accuracy. Spore-forming strains like Bacillus species offer inherent stability advantages, maintaining viability even in challenging storage conditions.[47]
Absence of Harmful Fillers
Scrutinize ingredient lists for unnecessary additives that may reduce efficacy or cause problems. Common concerns include:
- Microcrystalline cellulose (MCC): This common filler provides no nutritional value and may potentially reduce bioavailability
- Magnesium stearate: While generally recognized as safe, this flow agent can potentially interfere with absorption
- Silicon dioxide and other flow agents: Often unnecessary in well-formulated products
- Artificial colors, flavors, or sweeteners: Provide no benefit and may disrupt gut bacteria
MicroBiome Restore™ contains zero harmful fillers, using only the active probiotic strains, organic prebiotics, and pullulan capsule material.
Prebiotic Inclusion and Quality
True synbiotic formulations include prebiotics specifically selected to nourish their probiotic strains. Look for organic, diverse prebiotic sources rather than a single fiber type. The best prebiotic blends include multiple fiber types that feed different bacterial families, creating comprehensive ecosystem support.
Third-Party Testing and Certifications
Quality manufacturers invest in third-party testing to verify potency, purity, and absence of contaminants. Look for products tested by independent laboratories and carrying relevant certifications (GMP, NSF, or similar quality standards). This provides assurance that the product contains what the label claims without harmful adulterants.
Appropriate Storage and Handling
While many modern probiotics use shelf-stable strains that don't require refrigeration, storage conditions still matter. Products should be stored in cool, dry environments away from direct sunlight. Advanced capsule technologies like pullulan enhance stability, but proper storage ensures maximum bacterial viability through the product's expiration date.
Conclusion: Harnessing the Power of Synbiotic Synergy
The convergence of prebiotic and probiotic research has revealed what traditional fermented food cultures understood intuitively: beneficial bacteria thrive when provided with their preferred nourishment, and the health benefits multiply when these components work together. As we've explored throughout this guide, synbiotic combinations produce effects that exceed what either component achieves independently—strengthening gut barrier function, reducing systemic inflammation, modulating immune responses, supporting metabolic health, and even influencing mental wellness through the gut-brain axis.
The synbiotic approach is especially valuable during antibiotic recovery, when your depleted beneficial bacteria need both reinforcements and nourishment—learn more in our probiotics after antibiotics guide.
The scientific evidence base continues expanding rapidly, with 2024 and 2025 research providing increasingly sophisticated insights into mechanisms of action, optimal strain combinations, and therapeutic applications across diverse conditions. What remains clear is that gut microbiome health represents a fundamental pillar of overall wellness, influencing virtually every physiological system through complex bidirectional communication networks.
Multi-strain formulations containing both traditional probiotic species (Lactobacillus and Bifidobacterium) and resilient soil-based organisms (Bacillus species), combined with diverse organic prebiotic fibers, offer the most comprehensive approach to microbiome support. Advanced delivery technologies like pullulan capsules ensure these beneficial bacteria reach their intended destination alive and active, ready to colonize and produce the metabolites that drive health benefits.
MicroBiome Restore™ represents this evolution in synbiotic science—26 carefully selected strains working synergistically with 9 organic prebiotics, delivered in fermented pullulan capsules that provide oxygen barrier protection and delayed release while contributing their own prebiotic benefits. With zero harmful fillers and comprehensive third-party testing, this formulation addresses gut health from every angle: diversity of strains, quality of prebiotics, effectiveness of delivery, and purity of ingredients.
Whether you're seeking to address specific digestive complaints, support immune function, optimize metabolic health, or simply maintain the foundation of wellness that a balanced microbiome provides, the synbiotic approach offers science-backed benefits that continue accumulating with consistent use. Your gut microbiome took years to develop its current composition; comprehensive restoration requires patience and consistency. Yet the investment yields dividends across multiple dimensions of health—from the comfort of improved digestion to the profound systemic benefits of reduced inflammation, enhanced immunity, and optimal metabolic function.
The journey to optimal gut health begins with a single step. Make that step a comprehensive one by choosing synbiotic formulations that provide diverse bacterial strains, quality prebiotics, advanced delivery technology, and the purity your body deserves. Your trillions of microscopic allies are waiting for the support they need to keep you thriving.
References
- Yassine, F., Najm, A., & Bilen, M. (2025). The role of probiotics, prebiotics, and synbiotics in the treatment of inflammatory bowel diseases: an overview of recent clinical trials. Frontiers in Systems Biology, 5, 1561047. https://doi.org/10.3389/fsysb.2025.1561047
- Zhuang, K., Luo, H., Zeng, M., et al. (2025). Effects of probiotics, prebiotics, and synbiotics on gut microbiota in older adults: a systematic review and meta-analysis of randomized controlled trials. Nutrition Journal, 24, 147. https://doi.org/10.1186/s12937-025-01218-1
- Zaidi, A. Z., Moore, S. E., & Okala, S. G. (2021). Impact of maternal nutritional supplementation during pregnancy and lactation on the infant gut or breastmilk microbiota: A systematic review. Nutrients, 13(4), 1137. https://doi.org/10.3390/nu13041137
- Kirschner, S. K., Engelen, M. P., Haas, P., Bischoff, S. C., & Deutz, N. E. (2025). Short-chain fatty acid kinetics and concentrations are higher after inulin supplementation in young and older adults: a randomized trial. American Journal of Clinical Nutrition, 121(6), 1224-1235. https://doi.org/10.1016/j.ajcnut.2025.03.012
- Li, E., Wang, J., Guo, B., & Zhang, W. (2025). Effects of short-chain fatty acid-producing probiotic metabolites on symptom relief and intestinal barrier function in patients with irritable bowel syndrome: a double-blind, randomized controlled trial. Frontiers in Cellular and Infection Microbiology, 15, 1616066. https://doi.org/10.3389/fcimb.2025.1616066
- Payahoo, M., Amini-Salehi, E., et al. (2025). A systematic review of the effect of probiotics, prebiotics, and synbiotics on gut microbiota integrity markers in type 2 diabetes mellitus. Endocrinology Research and Practice. [Epub ahead of print]
- Popescu, F. D., et al. (2025). A review of the influence of prebiotics, probiotics, synbiotics, and postbiotics on the human gut microbiome and intestinal integrity. Nutrients. [Collection date 2025 Jun]. https://pmc.ncbi.nlm.nih.gov/articles/PMC12156228/
- BioPhysics Essentials. (2024). Hypromellose (HPMC) vs pullulan: Best probiotic capsules for gut health. Retrieved from https://biophysicsessentialsofficial.com/blogs/gut-check/hypromellose-hpmc-vs-pullulan-capsules-gut-health
- Zhuang, K., Luo, H., Zeng, M., et al. (2025). Effects of probiotics, prebiotics, and synbiotics on gut microbiota in older adults: a systematic review and meta-analysis of randomized controlled trials. Nutrition Journal, 24, 147. https://doi.org/10.1186/s12937-025-01218-1
- Sharma, M., et al. (2023). Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World Journal of Gastroenterology. https://pmc.ncbi.nlm.nih.gov/articles/PMC10130969/
- Salminen, S., Collado, M. C., Endo, A., et al. (2023). World Gastroenterology Organisation global guidelines: Probiotics and prebiotics. https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf
- Kayser, E., He, F., Nixon, S., et al. (2025). Enhancement of carbohydrate metabolism by probiotic and prebiotic intake promotes short-chain fatty acid production in the gut microbiome: a randomized, double-blind, placebo-controlled crossover trial. Bioscience of Microbiota, Food and Health, 89(8), 1191-1202. https://doi.org/10.1093/bbb/zbaf071
- Koh, A., De Vadder, F., Kovatcheva-Datchary, P., & Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell, 165, 1332-1345. https://doi.org/10.1016/j.cell.2016.05.041
- Singh, V., Lee, G., Son, H., et al. (2023). Butyrate producers, "The Sentinel of Gut": Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.1103836
- Li, E., Wang, J., Guo, B., & Zhang, W. (2025). Effects of short-chain fatty acid-producing probiotic metabolites on symptom relief and intestinal barrier function in patients with irritable bowel syndrome. Frontiers in Cellular and Infection Microbiology, 15, 1616066. https://doi.org/10.3389/fcimb.2025.1616066
- Liśkiewicz, P., et al. (2024). Short-chain fatty acids and their impact on human health. Polish Merck's Index. https://www.monz.pl/pdf-190750-114808
- Parada Venegas, D., et al. (2021). Short chain fatty acids (SCFAs): An overlooked therapy for IBD? eBioMedicine, 66, 103323. https://doi.org/10.1016/j.ebiom.2021.103323
- Mann, E. R., Lam, Y. K., & Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nature Reviews Immunology, 24, 577-595. https://doi.org/10.1038/s41577-024-01014-8
- Payahoo, M., et al. (2025). A systematic review of the effect of probiotics, prebiotics, and synbiotics on gut microbiota integrity markers in type 2 diabetes. Endocrinology Research and Practice. [Epub ahead of print]
- Asefa, G., et al. (2025). Postbiotics and their biotherapeutic potential for chronic disease. Frontiers in Microbiomes. https://doi.org/10.3389/frmbi.2025.1489339
- Awad, H. M., et al. (2025). Comparative in silico analyses between Lactiplantibacillus plantarum and Bifidobacterium longum concerning probiotic properties, anti-lipidemic, and anti-diabetic in vitro activities. BMC Microbiology, 25, 356. https://doi.org/10.1186/s12866-025-04062-9
- Pagnini, C., et al. (2025). Gut-microbiota-derived metabolites and probiotic strategies in colorectal cancer: Implications for disease modulation and precision therapy. Nutrients. [Collection date 2025 Aug]. https://pmc.ncbi.nlm.nih.gov/articles/PMC12348773/
- Stavropoulou, E., & Bezirtzoglou, E. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. https://pmc.ncbi.nlm.nih.gov/articles/PMC7230973/
- Kailash, C., et al. (2024). Understanding how pre- and probiotics affect the gut microbiome and metabolic health. American Journal of Physiology-Endocrinology and Metabolism. https://doi.org/10.1152/ajpendo.00054.2024
- Barengolts, E. (2016). Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocrine Practice, 22(10), 1224-1234. https://doi.org/10.4158/EP151157.RA
- Zhang, C., Zhang, Q., Zhang, X., et al. (2025). Effects of synbiotics surpass probiotics alone in improving type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clinical Nutrition, 44, 248-258. https://doi.org/10.1016/j.clnu.2024.11.042
- Vargas-Fernández, E. F., Lozano-Montoya, L., & Munguía-Batista, M. (2025). The effect of probiotics, prebiotics and synbiotics on gut microbial community profile in overweight and obese Latin American and Caribbean populations: a systematic review. Gut Microbiome, 6, e2. https://doi.org/10.1017/gmb.2024.12
- Mahadik, K. N., Agarwal, R., Patange, A., et al. (2025). Impact of a probiotic-fiber blend on body weight, metabolic regulation, and digestive function in obese adults: A randomized, placebo-controlled, multicentric trial. Cureus, 17(4), e82613. https://doi.org/10.7759/cureus.82613
- Cardinelli, C. S., & Sala, P. C. (2024). Probiotics, prebiotics, synbiotics and other microbiome-based innovative therapeutics to mitigate obesity and enhance longevity via the gut-brain axis. Microbiome Research Reports. https://doi.org/10.1016/j.mrr.2024.05
- Awad, H. M., et al. (2025). Comparative in silico analyses between Lactiplantibacillus plantarum and Bifidobacterium longum concerning probiotic properties. BMC Microbiology, 25, 356. https://doi.org/10.1186/s12866-025-04062-9
- Wiertsema, S. P., van Bergenhenegouwen, J., Garssen, J., & Knippels, L. M. J. (2021). The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients, 13(3), 886. https://doi.org/10.3390/nu13030886
- Li, M., et al. (2025). Frontiers in recent development of probiotic Bifidobacteria for treating human diseases. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2021.770248
- Shandilya, S., et al. (2024). The anti-inflammatory and curative exponent of probiotics: A comprehensive and authentic ingredient for the sustained functioning of major human organs. Nutrients. https://pmc.ncbi.nlm.nih.gov/articles/PMC10893534/
- Park, J. E., et al. (2023). Lactobacillus rhamnosus and Bifidobacterium longum alleviate colitis and cognitive impairment in mice by regulating IFN-γ to IL-10 and TNF-α to IL-10 expression ratios. Scientific Reports. https://doi.org/10.1038/s41598-021-00096-x
- Collado, M. C., Meriluoto, J., & Salminen, S. (2008). Adhesion and aggregation properties of probiotic and pathogen strains. European Food Research and Technology, 226, 1065-1073. https://doi.org/10.1007/s00217-007-0632-x
- Jeong, K., Kim, M., Jeon, S. A., Kim, Y. H., & Lee, S. (2024). Efficacy of Bifidobacterium longum and Lactobacillus plantarum (NVP-1703) in children with allergic rhinitis: A randomized controlled trial. Journal of Korean Medical Science, 39, e266. https://doi.org/10.3346/jkms.2024.39.e266
- Yassine, F., Najm, A., & Bilen, M. (2025). The role of probiotics, prebiotics, and synbiotics in the treatment of inflammatory bowel diseases. Frontiers in Systems Biology, 5, 1561047. https://doi.org/10.3389/fsysb.2025.1561047
- Cao, Y.-N., et al. (2018). Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi Journal of Gastroenterology, 24(1), 59-66. https://pmc.ncbi.nlm.nih.gov/articles/PMC5848327/
- Bravo, J. A., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences, 108(38), 16050-16055. https://doi.org/10.1073/pnas.1102999108
- Liang, S., Wu, X., & Jin, F. (2018). Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Frontiers in Integrative Neuroscience, 12, 33. https://doi.org/10.3389/fnint.2018.00033
- Vivion. (2025). Pullulan capsules 101: Manufacturer's essential guide. Retrieved from https://vivion.com/pullulan-capsules-101-the-essential-guide-for-manufacturers/
- Capsuline. (2025). Delayed-release capsules ideal for probiotic and enzyme supplements. Retrieved from https://capsuline.com/blogs/capsuline-blog/delayed-release-capsules-for-probiotics-and-enzymes
- Mohammadi-Jam, S., & Burnett, D. J. (2023). Towards a novel pullulan and pullulan-enteric hard capsules: Study of drug release and crucial capsule features. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2023.128417
- Meng, Z., He, Q., Mu, L., et al. (2025). Pullulan-spermine enhance the tolerance of probiotics and immune stimulation of macrophages. International Journal of Biological Macromolecules, 287, 138417. https://doi.org/10.1016/j.ijbiomac.2024.138417
- Timmerman, H. M., Koning, C. J., Mulder, L., Rombouts, F. M., & Beynen, A. C. (2004). Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. International Journal of Food Microbiology, 96(3), 219-233. https://doi.org/10.1016/j.ijfoodmicro.2004.05.012
- Barba-Vidal, E., et al. (2017). Combinations of a probiotic and a Bacillus sp. endospore as a strategy to enhance gut health in weaned piglets. Beneficial Microbes, 8(5), 739-751. https://doi.org/10.3920/BM2016.0211
- World Gastroenterology Organisation. (2023). World Gastroenterology Organisation global guidelines: Probiotics and prebiotics. Retrieved from https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf
- BioPhysics Essentials. (2024). Benefits of trace minerals for gut health. Retrieved from https://biophysicsessentialsofficial.com/blogs/gut-check/benefits-trace-minerals-gut-health









Share and get 15% off!
Simply share this product on one of the following social networks and you will unlock 15% off!