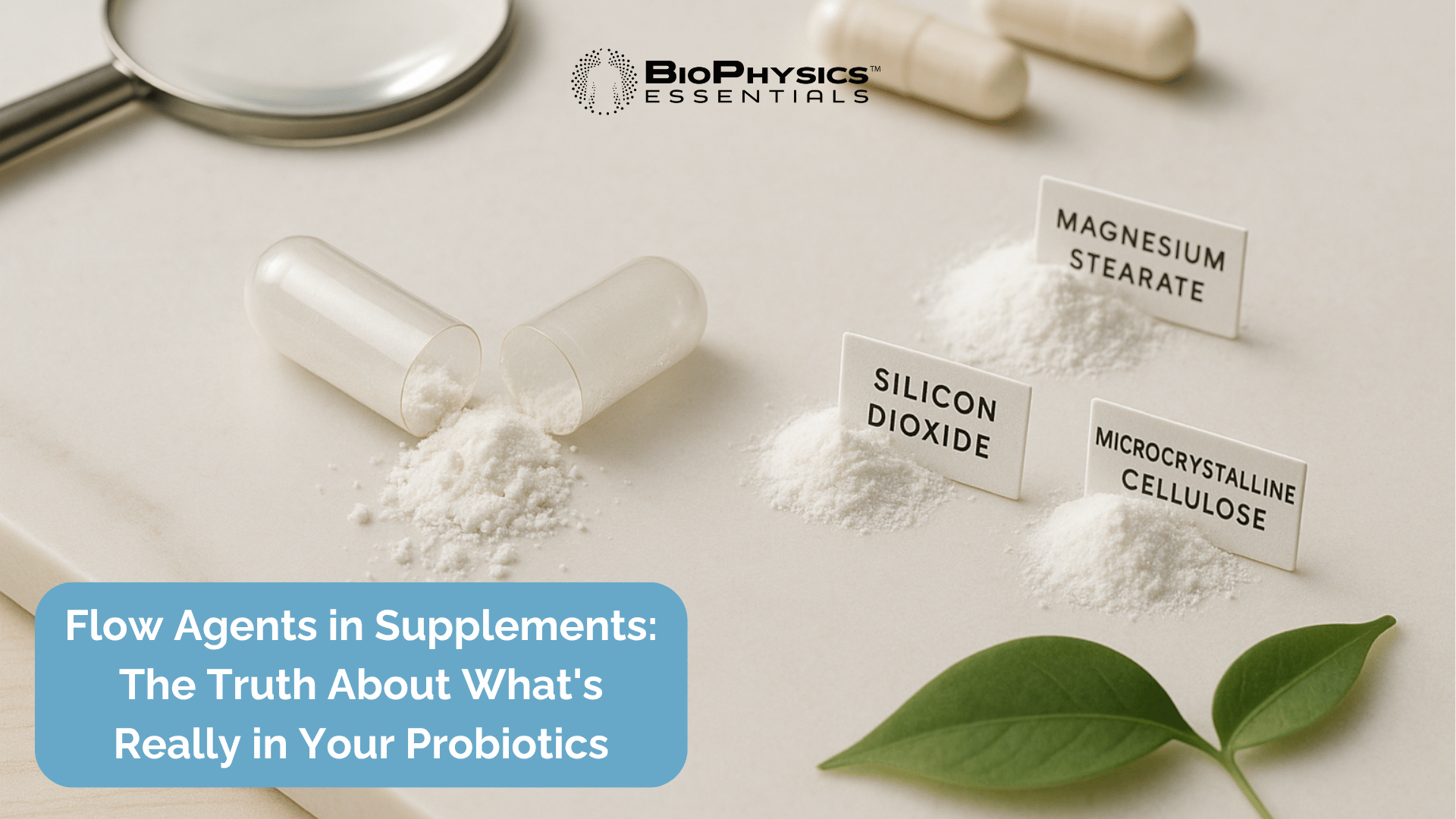
What Are Flow Agents in Supplements? Understanding Hidden Fillers in Your Probiotics
When you pick up a probiotic supplement, you're likely focused on the beneficial bacteria strains and their promised health benefits. But have you ever stopped to examine the "other ingredients" list? Hidden among the active components are substances called flow agents—manufacturing aids designed to make production faster and cheaper, not to improve your health. Understanding what these ingredients are, why they're used, and whether they belong in your supplements is crucial for making informed choices about your gut health.
Key Points
- Flow agents like silicon dioxide and magnesium stearate are manufacturing aids added to supplements to improve powder flow during production
- Silicon dioxide has been shown to damage intestinal microvilli and impair nutrient absorption at realistic exposure levels
- These excipients occupy valuable capsule space that could contain beneficial prebiotics, probiotics, or minerals
- Clean-label formulations eliminate flow agents to maximize therapeutic ingredient density
- Premium probiotics like MicroBiome Restore prove that supplements can be manufactured without compromising on quality or purity
What Are Flow Agents?
Flow agents, also known as glidants or anti-caking agents, are substances added to supplement formulations to improve the flow characteristics of powder blends during manufacturing. [1] Think of them as the lubricants of the supplement world—they help powders move smoothly through high-speed capsule filling machines, prevent clumping during storage, and ensure consistent fill weights in every capsule or tablet.
From a manufacturing perspective, flow agents solve real technical challenges. Powder blends containing probiotics, minerals, and botanical ingredients naturally have poor flowability due to cohesive forces between fine particles. [2] Without flow agents, these powders stick to equipment, create production bottlenecks, and result in inconsistent dosing. High-speed automatic capsule filling machines operating at 40,000-250,000 capsules per hour require excellent powder flow to maintain quality and efficiency.
However, the question health-conscious consumers should ask isn't whether flow agents serve a manufacturing purpose—it's whether that purpose justifies their inclusion when alternatives exist and when these substances may compromise the very health benefits you're seeking from your supplements.
Common Types of Flow Agents in Supplements
Silicon Dioxide (Silica)
Silicon dioxide is perhaps the most ubiquitous flow agent in the supplement industry, appearing in everything from multivitamins to probiotics. [3] Also listed as silica, silicon dioxide, or simply E551 on European labels, this white powdery substance prevents ingredients from clumping together and improves powder flow during encapsulation.
The industry claims silicon dioxide is safe because it's found naturally in foods and passes through the digestive system largely unchanged. However, recent research paints a more concerning picture. A 2018 study examining food-grade silicon dioxide at physiologically relevant doses found that nanoparticle exposure decreased the number of intestinal microvilli—the tiny finger-like projections that maximize nutrient absorption surface area in your gut. [4]
The same study documented that silicon dioxide significantly impaired nutrient transport, with iron transport decreasing at all chronic dose levels and fatty acid uptake declining with low-dose chronic exposure. Perhaps most alarming, transepithelial electrical resistance decreased significantly after just two days of chronic exposure, indicating compromised intestinal barrier function—the "leaky gut" phenomenon that's increasingly recognized as a root cause of systemic inflammation.
For individuals taking probiotic supplements specifically to heal their gut lining and improve nutrient absorption, consuming a substance that damages the very tissues you're trying to repair seems counterproductive at best and actively harmful at worst.
Magnesium Stearate
Magnesium stearate is a salt composed of magnesium and stearic acid that functions as a lubricant in supplement manufacturing. [5] Added at concentrations of 0.5-1% in most formulations, it reduces friction between powder particles and equipment, preventing ingredients from sticking to capsule filling machines and ensuring tablets can be compressed and ejected smoothly.
The safety debate around magnesium stearate has raged for years in natural health communities. Proponents point to extensive safety data showing no toxicity at typical supplement doses, with animal studies establishing a no-effect level thousands of times higher than human exposure. [6] A 2017 genotoxicity study battery returned negative results, confirming lack of DNA-damaging potential.
However, the more nuanced concern isn't acute toxicity but rather opportunity cost and potential subtle effects. In a size 00 capsule with approximately 750mg capacity, if 50mg (6.7%) is occupied by magnesium stearate and other excipients, that's 50mg unavailable for beneficial probiotic strains, prebiotic fibers, or trace minerals. [7] For formulations prioritizing maximum therapeutic ingredient density, this represents wasted potential.
Recent research on gut microbiome modulation by stearic acid adds another dimension to consider. A 2021 Nature journal study found high stearic acid diets dramatically enriched Akkermansia muciniphila and altered short-chain fatty acid production. [8] While the doses from dietary sources far exceed supplement-derived magnesium stearate, the principle remains: why introduce any substance with uncertain microbiome effects when the entire point of taking probiotics is to optimize gut bacterial balance?
Microcrystalline Cellulose
Microcrystalline cellulose (MCC) deserves mention because while technically a filler rather than a flow agent, it's often used in conjunction with flow agents and serves similar manufacturing convenience purposes. MCC is refined wood pulp—yes, literally processed tree fiber—added to supplements primarily to occupy space when active ingredients don't fill the capsule completely. [9]
From a safety standpoint, MCC is genuinely inert. It passes through the digestive system unchanged, doesn't bind nutrients or interfere with absorption, and has no known toxicity. The European Food Safety Authority confirmed MCC "remained intact until excreted" in its 2018 re-evaluation. [10]
The legitimate objection to MCC isn't safety but value. Why would you want wood pulp occupying 10-30% of your probiotic capsule when that space could contain mineral-rich sea vegetables, medicinal mushrooms, or additional probiotic strains? MCC contributes nothing therapeutic—it's purely a cheap bulking agent that makes manufacturing easier and more profitable for supplement companies.
For a deeper examination of MCC's role in supplements, see our comprehensive article on microcrystalline cellulose safety concerns.
Beyond flow agents like magnesium stearate and silica, many supplements also contain titanium dioxide as a whitening agent. The European Union banned this additive in 2022 after safety authorities determined titanium dioxide can no longer be considered safe due to genotoxicity concerns.
How Flow Agents Work in Supplement Manufacturing
Improving Production Efficiency
To understand why flow agents are so prevalent, you need to appreciate the economics and physics of modern supplement manufacturing. High-speed automatic capsule filling equipment operates at speeds that would be impossible without excellent powder flowability. [11] These machines use dosing mechanisms—dosator pins or dosing discs—that must dip into powder beds, fill with precise amounts, and deposit into capsule shells thousands of times per hour.
Poor powder flow creates multiple problems: inconsistent fill weights (some capsules underfilled, others overfilled), equipment fouling (powder sticking to machinery requiring frequent shutdowns for cleaning), production delays (slower speeds to manage difficult materials), and increased rejection rates (capsules failing quality control). Each of these issues costs money, and in high-volume manufacturing, even small inefficiencies compound dramatically.
Flow agents solve these problems elegantly. By coating individual powder particles and reducing cohesive forces, they transform sticky, clumping powders into free-flowing materials that glide smoothly through equipment. [2] A typical 0.5-1% addition of magnesium stearate or silicon dioxide can increase production speeds by 30-50% while dramatically reducing equipment maintenance requirements.
Preventing Ingredient Clumping
Beyond manufacturing efficiency, flow agents serve a storage stability function. Many probiotic and prebiotic ingredients are hygroscopic—they absorb moisture from the environment. [12] Without anti-caking agents, these ingredients gradually clump together during storage, creating hard masses that won't properly disperse when consumed.
Silicon dioxide is particularly effective at preventing moisture-induced caking because its high surface area absorbs water while maintaining free-flowing properties. This extends shelf life and ensures the product pours smoothly from bottles even months after manufacturing.
However, the question remains: are these benefits worth the trade-offs? Alternative approaches exist for manufacturers willing to accept slightly higher costs and slower production speeds. Climate-controlled manufacturing environments (35-60% relative humidity), modified filling equipment, smaller batch sizes, and careful ingredient selection can minimize or eliminate flow agent requirements entirely.
The Safety Debate: Are Flow Agents Harmful?
Regulatory Standards and GRAS Status
The FDA considers most common flow agents "Generally Recognized as Safe" (GRAS) for use in food and supplements. [13] This designation means that qualified experts have reviewed available safety data and concluded there's "reasonable certainty of no harm" under intended use conditions. GRAS status can be achieved through FDA notification, affirmation through rulemaking, or until recently, self-affirmation by companies' own expert panels.
However, GRAS status has significant limitations that health-conscious consumers should understand. The threshold is preventing acute harm, not optimizing wellness. GRAS evaluations consider acute toxicity, organ toxicity, and genotoxicity, but they don't evaluate long-term health optimization, subtle metabolic effects, inflammatory potential, microbiome impacts, or cumulative exposures from multiple sources. [14]
The regulatory framework also creates blindspots. Until March 2025 when HHS announced plans to eliminate self-affirmed GRAS, companies could add thousands of ingredients to supplements without FDA knowledge or review. [15] While this loophole is closing, it illustrates that GRAS status should be understood as "probably won't cause obvious harm" rather than "definitely optimal for health."
The divergence between regulatory bodies further undermines confidence in universal safety claims. The European Food Safety Authority banned titanium dioxide (a common colorant and flow agent) in 2022 after concluding it could "no longer be considered safe" due to inability to exclude genotoxicity. [16] Yet the FDA continues permitting its use in the United States. Both agencies reviewed the same scientific evidence and reached opposite conclusions.
Potential Health Concerns
The peer-reviewed literature on flow agent safety presents a mixed but increasingly concerning picture:
Silicon Dioxide Intestinal Damage: The most robust evidence of harm comes from silicon dioxide research. The 2018 PMC study found that food-grade silica at estimated daily intake levels (35mg) caused measurable intestinal damage including decreased microvilli density, impaired nutrient transport, compromised barrier function, and increased inflammatory markers (IL-8 expression increased 3.28-fold, TNF-α increased 9.61-fold). [4]
These aren't theoretical concerns from mega-doses in laboratory animals—these are effects demonstrated at realistic human exposure levels in sophisticated in vitro models. The nanoparticle fraction (particles below 100nm) drives much of the toxicity, and food-grade silicon dioxide contains significant nanoparticle content.
Biofilm Formation Concerns: Online health communities frequently cite concerns about magnesium stearate creating "biofilms" in the intestines that block nutrient absorption. While the biofilm claim lacks scientific support (research actually shows stearic acid inhibits biofilm formation), the dissolution rate concern has more merit. [17] Studies show magnesium stearate can slow how quickly tablets dissolve without necessarily reducing total absorption. For most drugs and nutrients this distinction doesn't matter clinically, but it illustrates that supposedly inert excipients do interact with active ingredients in measurable ways.
Microbiome Modulation: The emerging science of gut microbiome interactions reveals that almost everything we ingest affects bacterial populations in some way. While dietary sources of stearic acid dwarf supplement-derived amounts (5,900-8,800mg daily from food versus less than 20mg from supplements), the principle matters: if you're taking probiotics specifically to optimize gut bacterial balance, why introduce any substance with uncertain microbiome effects? [8]
Flow Agents vs. Fillers: Understanding the Difference
The supplement industry uses various categories of excipients—inactive ingredients that serve manufacturing rather than therapeutic purposes. Understanding the distinctions helps consumers make informed choices:
Flow Agents/Glidants: Substances like silicon dioxide and magnesium stearate that improve powder flowability and prevent clumping. Typically used at 0.5-2% of formulation weight. Primary purpose is facilitating high-speed manufacturing.
Fillers/Bulking Agents: Ingredients like microcrystalline cellulose or rice flour that occupy space when active ingredients don't fill the capsule. Can comprise 10-50% of formulation. Primary purpose is creating physical volume for processing and handling.
Binders: Substances that hold tablet ingredients together during compression. Examples include hydroxypropyl methylcellulose (HPMC), povidone, or gums. Essential for tablet formation but generally unnecessary for capsule formulations.
Disintegrants: Ingredients that help tablets break apart in the digestive system. Ironically, microcrystalline cellulose can function as both a filler and disintegrant depending on the formulation.
Colorants and Flavorings: Purely cosmetic additives like titanium dioxide (white color) or various FD&C dyes. These serve no functional purpose beyond making products visually appealing.
The key insight is that none of these excipient categories provide health benefits—they exist solely for manufacturing convenience, cost reduction, or cosmetic appeal. Every milligram occupied by excipients represents lost opportunity for beneficial ingredients.
The Opportunity Cost: What You're Missing
Perhaps the most compelling argument against flow agents and fillers isn't direct harm but rather wasted potential. Capsule volumes are fixed constraints: a size 00 capsule holds approximately 0.91-0.95 mL (roughly 570-1,140mg depending on powder density). [18] Every milligram of inactive ingredients is a milligram unavailable for active therapeutic components.
Consider a typical mainstream probiotic formulation versus a clean-label alternative:
Conventional Probiotic Capsule:
- 500mg probiotic blend (100 billion CFU)
- 150mg microcrystalline cellulose (filler)
- 30mg silicon dioxide (flow agent)
- 20mg magnesium stearate (lubricant)
Clean-Label Formulation:
- 400mg probiotic blend (15 billion CFU, more strain diversity)
- 800mg organic prebiotic blend (acacia, inulin, sea vegetables)
- 50mg medicinal mushroom extract (immune support)
- 0mg excipients
The difference is stark. The conventional formulation dedicates a large amount of capsule space to inactive ingredients that contribute nothing therapeutic. The clean formulation uses every milligram purposefully, incorporating diverse prebiotics that feed beneficial bacteria, trace minerals that support metabolic function, and additional probiotic strains for greater bacterial diversity.
This opportunity cost becomes even more significant when you consider that most people take supplements daily for months or years. Over a year of daily probiotic use, conventional formulations deliver approximately 18 grams of wood pulp, silicon dioxide, and magnesium stearate versus 18 grams of additional therapeutic ingredients in clean formulations.
Why Manufacturers Use Flow Agents (And Why Some Don't)
Understanding manufacturer incentives provides crucial context for evaluating supplement quality. Flow agents and fillers offer compelling business advantages:
Cost Reduction: High-speed automated production with fewer shutdowns, equipment cleaning, and maintenance dramatically lowers per-unit manufacturing costs. Excipients themselves cost pennies compared to premium probiotic strains or exotic botanicals. [19]
Production Scalability: Equipment running at 100,000+ capsules per hour enables enormous production volumes from single facilities. This economy of scale allows mass-market pricing and retail distribution.
Consistent Quality Control: Excellent powder flow ensures every capsule contains nearly identical fill weights, reducing rejection rates and quality control failures. This consistency matters for regulatory compliance and consumer satisfaction.
Extended Shelf Life: Anti-caking agents prevent clumping during storage, maintaining product appearance and pourability for years. This enables longer distribution chains and retail shelf presence.
Simplified Formulation: Standardized excipient use means manufacturers can rapidly develop new products using proven recipes and equipment settings rather than optimizing each unique formulation.
However, these advantages primarily benefit manufacturers and retailers, not consumers. The real question is whether companies willing to accept higher costs and more complex production can deliver superior products by eliminating excipients entirely.
The answer is unequivocally yes. Clean-label manufacturers prove that alternatives exist:
Small-Batch Production: Semi-automatic equipment handling 15,000-25,000 capsules per hour can manage difficult powders without extensive excipient use. Lower speeds allow more precise control and accommodation of challenging materials.
Climate-Controlled Manufacturing: Maintaining 35-60% relative humidity environments prevents hygroscopic ingredients from absorbing moisture and clumping, reducing anti-caking agent requirements. [12]
Modified Equipment: Specialized capsule filling technologies like flush-filling systems and intermittent fillers with suction bowl features can handle complex powders by removing air and increasing powder density through mechanical means rather than chemical additives.
Strategic Ingredient Selection: Choosing probiotic strains and prebiotic fibers with complementary flow properties minimizes manufacturing challenges. Certain ingredients naturally improve blend flowability without requiring separate flow agents.
Capsule Format Advantages: Capsules inherently require fewer excipients than tablets since they avoid the extreme compression forces that cause most manufacturing challenges. This is why virtually all premium clean-label probiotics use capsules rather than tablets. [20]
The trade-offs are real: slower production speeds, smaller batch sizes, higher per-unit costs, and more complex quality control. Premium clean-label probiotics typically cost 50-70% more than mass-market alternatives. But for consumers prioritizing ingredient purity and maximum therapeutic density, this premium delivers genuine value rather than just marketing spin.
How to Identify Flow Agents on Supplement Labels
Supplement labels list ingredients in two sections: the Supplement Facts panel showing active ingredients with their amounts, and the "Other Ingredients" section listing inactive ingredients (excipients). [21] Flow agents and fillers always appear in this secondary list, often in small print below the main panel.
Common flow agent names to watch for:
- Silicon dioxide
- Silica
- Magnesium stearate
- Stearic acid
- Calcium stearate
- Vegetable magnesium stearate (same as magnesium stearate, just from plant sources)
Common filler names:
- Microcrystalline cellulose (MCC)
- Cellulose
- Rice flour or rice powder
- Dicalcium phosphate
- Calcium carbonate (when in large amounts)
Other excipients to consider:
- Titanium dioxide (colorant, banned in EU)
- FD&C dyes (artificial colors)
- Talc (anti-caking, potential asbestos contamination risk)
- Hydrogenated oils
When evaluating probiotic supplements specifically, ask yourself: does every ingredient serve a therapeutic purpose? If the "Other Ingredients" list is longer than the active ingredients, that's a red flag indicating formulation priorities favor manufacturing convenience over your health benefits.
For consumers seeking truly clean formulations, look for products that explicitly state "no fillers," "no flow agents," or "no excipients." Better yet, examine the actual ingredient list—clean-label products will show only active ingredients (probiotic strains, prebiotic fibers, minerals) plus the capsule material itself.
Clean-Label Alternatives: MicroBiome Restore's Approach
At BioPhysics Essentials, we formulated MicroBiome Restore based on a uncompromising philosophy: every single milligram in the capsule should serve a beneficial purpose. This "all-in" approach eliminates all conventional excipients in favor of maximum therapeutic ingredient density.
The formulation delivers 26 total bacterial strains (21 probiotic plus 5 soil-based organisms), 9 certified organic prebiotics, and 80+ trace minerals from bioactive leonardite—all providing a minimum 15 billion CFU per two-capsule serving. Zero flow agents. Zero fillers. Zero wasted space.
Instead of silicon dioxide and magnesium stearate, MicroBiome Restore incorporates functional ingredients that enhance probiotic efficacy:
Organic Sea Vegetables: Norwegian kelp, bladderwrack, and oarweed provide mineral-rich alginate fiber that feeds beneficial bacteria while delivering iodine and trace minerals. These certified organic marine botanicals occupy the space other formulations waste on cellulose fillers.
Bioactive Leonardite: Fossilized peat-derived fulvic acid and trace minerals support heavy metal detoxification while providing bacterial nutrition. This replaces the inert silicon dioxide other manufacturers use as flow agents.
Medicinal Mushrooms: Maitake mushroom extract stimulates beneficial bacteria growth and encourages short-chain fatty acid production while supporting immune function. Learn more about maitake's gut health benefits.
Acacia Senegal Gum: This soluble prebiotic fiber is gentle on sensitive digestive systems and low-FODMAP friendly, providing slow-fermenting nutrition for probiotics. Read our detailed guide on acacia senegal gum benefits.
Pullulan Capsules: Even the capsule material serves dual purpose. Pullulan is a plant-based polysaccharide created through fermentation that functions as both a protective shell and a prebiotic substrate. Research shows pullulan is selectively fermented by beneficial Lactobacillus and Bifidobacterium species, effectively making the capsule itself functional nutrition rather than inert packaging. [22]
This approach requires accepting higher manufacturing costs, slower production speeds, and climate-controlled facilities. But the result is a formulation where literally every ingredient contributes to gut ecosystem restoration rather than just manufacturing convenience.
For those seeking comprehensive digestive support, our Gut Essentials Protocol combines MicroBiome Restore with X-Cellerator Full Spectrum Minerals for complete microbiome and metabolic support.
Making Informed Supplement Choices
Navigating supplement quality requires moving beyond marketing claims to evaluate actual formulations critically. Here's how to make informed choices:
Read Every Ingredient: Don't stop at the Supplement Facts panel—examine the complete "Other Ingredients" list. Long excipient lists suggest formulation priorities favor manufacturing over efficacy. [21]
Question Everything: Ask why each ingredient exists. If a probiotic contains rice flour or magnesium stearate, those serve manufacturing purposes, not your gut health. Premium formulations justify every component therapeutically.
Prioritize Strain Diversity: Multi-strain probiotics offer advantages over single-strain products, but only if the formulation isn't diluted with fillers. More strains packed into clean formulations provide greater bacterial diversity than fewer strains surrounded by excipients.
Consider Capsule Material: Gelatin capsules contain 13-16% moisture and risk cross-linking reactions. HPMC (hydroxypropyl methylcellulose) offers lower moisture content but remains semi-synthetic. Pullulan capsules provide superior oxygen barrier protection (300x better than HPMC) and prebiotic properties. [22]
Evaluate CFU Counts Realistically: Higher CFU numbers don't matter if formulations include substances that damage gut lining or if excipients reduce viable bacterial delivery. A clean-label 15 billion CFU product may outperform a conventional 50 billion CFU product loaded with fillers.
Accept Premium Pricing for Real Value: Clean-label formulations cost more to manufacture. If a probiotic is priced identically to mass-market alternatives, it likely uses similar manufacturing shortcuts. Premium pricing (typically $40-60 monthly) for genuinely clean formulations reflects real production costs, not just marketing markup.
Check for Prebiotic Integration: The best probiotic formulations include prebiotics to feed beneficial bacteria. This synbiotic approach maximizes colonization and therapeutic effect. Learn more about choosing the best prebiotics.
Understanding Excipient-Free Manufacturing Challenges
To appreciate why clean-label supplements command premium pricing, it's worth understanding the technical challenges manufacturers face when eliminating flow agents and fillers.
Hygroscopic Ingredient Management: Many probiotic strains and prebiotic fibers naturally absorb moisture from the environment, causing clumping, caking, and degraded flowability. [12] Without silicon dioxide or other anti-caking agents, manufacturers must invest in climate-controlled production facilities maintaining precise humidity levels (35-60% RH). This requires expensive HVAC systems, environmental monitoring, and careful batch scheduling.
Equipment Modifications: Standard high-speed capsule filling machines assume excellent powder flow enabled by conventional excipients. Clean-label formulations require specialized equipment—flush-filling technology, intermittent capsule fillers with modified dosing mechanisms, or semi-automatic systems that trade speed for precision. [11]
Batch Size Constraints: Smaller production runs maintain better control over challenging materials but increase per-unit costs significantly. Where conventional manufacturers might produce 500,000 units in a single run, clean-label producers may limit batches to 50,000-100,000 units.
Quality Control Complexity: Without excipients providing consistency, every batch requires more extensive testing to verify fill weight uniformity, dissolution characteristics, and probiotic viability. This adds labor costs and potentially increases rejection rates.
Ingredient Sourcing Challenges: Premium organic prebiotics, certified clean mineral sources, and specific probiotic strains cost substantially more than commodity ingredients. When you're not diluting formulations with cheap fillers, raw material costs dominate production economics.
Storage and Distribution Requirements: Products without anti-caking agents may be more sensitive to humidity and temperature fluctuations during warehousing and shipping. This necessitates better packaging (desiccants, barrier materials) and potentially refrigerated distribution chains.
These challenges are real, not imaginary. However, they're solvable for manufacturers prioritizing quality over profit margins. The question isn't whether clean-label manufacturing is possible—it demonstrably is—but whether companies choose to accept lower margins and more complex operations in service of genuinely superior products.
The Regulatory Landscape: What's Changing
The supplement industry regulatory environment is evolving in ways that favor transparency and may eventually restrict some controversial excipients:
Elimination of Self-Affirmed GRAS: In March 2025, HHS Secretary announced plans to end the self-affirmation pathway for GRAS ingredients, requiring all safety determinations be submitted to FDA for review. [15] This closes a major loophole where companies added thousands of ingredients without regulatory oversight. While implementation remains pending, this represents the most significant regulatory strengthening in decades.
International Regulatory Divergence: The EU's 2022 ban on titanium dioxide in food creates precedent for restricting excipients based on inability to establish safety rather than requiring proof of harm. [16] This precautionary principle may eventually influence US regulations, particularly for ingredients serving purely cosmetic functions.
Clean Label Market Pressure: Consumer demand for transparency is driving change faster than regulatory action. Industry data shows 81% of shoppers consider clean labels important, with 59% willing to pay premiums for quality ingredients. [23] This market pressure incentivizes manufacturers to reformulate even absent regulatory requirements.
Increased Scrutiny of Nanoparticles: Research documenting silicon dioxide nanoparticle toxicity may eventually prompt regulatory review of anti-caking agent safety limits. The EFSA's 2018 re-evaluation acknowledged inability to exclude genotoxicity concerns for certain particle sizes. [4]
Talc Testing Requirements: FDA proposed mandatory testing of talc-containing products in December 2024 following documentation of asbestos contamination. [24] While this addresses cosmetics primarily, the precedent may extend to dietary supplement anti-caking agents.
These regulatory shifts suggest the industry is slowly moving toward greater accountability and transparency. However, consumers shouldn't wait for regulatory mandates to demand better formulations—voting with purchasing dollars accelerates change faster than legislative processes.
Beyond Flow Agents: Other Excipients to Avoid
While flow agents represent a primary concern, several other excipient categories warrant consumer scrutiny:
Titanium Dioxide (TiO2): Used purely for cosmetic white coloring, titanium dioxide has been banned in EU foods since 2022 after EFSA determined it could "no longer be considered safe" due to genotoxicity concerns. [16] Yet it remains legal in US supplements. Since TiO2 provides zero health benefits, its presence signals formulation priorities favor appearance over safety.
Talc: An anti-caking agent with documented asbestos contamination history. A 2021 American Journal of Public Health investigation found that cosmetic talc "is not and never was asbestos-free" despite industry claims. [24] While pharmaceutical-grade USP talc theoretically meets higher standards, testing method limitations and geological realities (talc and asbestos naturally co-occur) make any talc inclusion questionable.
Artificial Colorants: FD&C dyes serve only cosmetic purposes yet carry potential health concerns ranging from allergic reactions to hyperactivity in children. Premium supplements have no need for artificial colors.
Hydrogenated Oils: Sometimes used as tablet lubricants, hydrogenated oils contain trans fats that should be avoided. Their presence indicates outdated formulation practices.
Carrageenan: A seaweed-derived thickener associated with intestinal inflammation in some studies. While regulatory agencies generally consider it safe, the inflammatory potential makes it problematic for gut health products specifically.
The principle remains consistent: premium formulations justify every ingredient therapeutically rather than including substances solely for manufacturing convenience, cost reduction, or cosmetic appeal.
Probiotic-Specific Considerations: How Excipients Affect Bacterial Viability
For probiotic supplements specifically, excipient choices affect more than just capsule space—they can impact bacterial survival during manufacturing, storage, and gastrointestinal transit.
Compression Stress: Studies on Lactobacillus rhamnosus GG demonstrate that compression forces during tablet manufacturing cause cell wall and membrane damage, with bacterial survival decreasing linearly with pressure. [25] This is one reason premium probiotics use capsules rather than tablets—capsule filling avoids the extreme mechanical stress of tablet compression that kills bacteria even when protective excipients are present.
Moisture Exposure: Gelatin capsules' high moisture content (13-16%) can transfer water to hygroscopic probiotic powders, reducing viability during storage. Hard gelatin can also undergo cross-linking reactions from UV light, high humidity, or reactions with ingredients, potentially preventing proper dissolution and probiotic release. [26]
Oxygen Protection: Oxygen-sensitive probiotic strains—particularly Bifidobacterium species and some Lactobacillus strains—suffer viability loss when exposed to air. Research shows pullulan capsules provide 300-fold better oxygen barrier protection compared to HPMC, dramatically extending shelf life for vulnerable strains. [22]
Storage Stability: Studies demonstrate that elastic filler-binders protect probiotics during storage by cushioning bacteria from mechanical stress. However, the same protective effect can be achieved through strategic formulation with functional ingredients (prebiotics, minerals) rather than inert fillers. [25]
Gastric Acid Survival: Microencapsulation studies show dramatic viability improvements when probiotics are protected from stomach acid. Encapsulated probiotics maintained 86-93% viability versus significant die-off in unprotected formulations. [27] Advanced capsule technologies like delayed-release HPMC or pullulan provide acid protection without requiring separate microencapsulation.
The evidence suggests that excipients do affect probiotic performance, but the effect depends critically on excipient type, manufacturing process, and storage conditions. More importantly, proper capsule material selection and protective technologies matter far more than excipient presence or absence. Clean-label formulations using pullulan capsules, appropriate prebiotic co-ingredients, and careful manufacturing can deliver superior probiotic viability compared to conventional formulations relying on synthetic protective excipients.
The Cost-Benefit Analysis: Is Clean Label Worth the Premium?
Clean-label probiotic supplements typically cost $40-60 monthly compared to $15-30 for mass-market alternatives—a 50-100% premium. Is this additional cost justified?
What You're Paying For:
- Ingredient Density: Every milligram serves therapeutic purpose rather than manufacturing convenience. Over a year, this translates to consuming approximately 18 grams additional beneficial ingredients versus 18 grams of wood pulp and lubricants.
- Strain Diversity: Clean formulations pack more probiotic strains into the same capsule size. MicroBiome Restore's 26 strains versus typical 5-10 strains in conventional products provides greater microbiome diversity.
- Organic Certification: Premium ingredients like certified organic sea vegetables, sustainably sourced minerals, and specialty prebiotics cost substantially more than commodity fillers.
- Superior Capsule Technology: Pullulan capsules cost 2-3x more than gelatin but provide measurably better oxygen protection and prebiotic benefits.
- Small-Batch Quality: Lower production volumes with more careful monitoring ensure consistent quality but increase per-unit costs.
What You're Avoiding:
- Silicon dioxide's documented intestinal damage and nutrient absorption impairment
- Microcrystalline cellulose occupying 10-30% of capsule space
- Talc's potential asbestos contamination
- Titanium dioxide banned in EU but still legal in US products
- Mystery ingredients from self-affirmed GRAS substances
The value equation depends on individual priorities. If supplement use is casual—trying probiotics to see if they help—mass-market products provide affordable entry points. But for individuals seriously committed to gut health restoration, dealing with digestive conditions, or prioritizing ingredient purity, the premium for clean formulations delivers genuine value rather than just marketing appeal.
Consider also that many people consume multiple supplements daily. If you're taking probiotics, multivitamins, minerals, and specialized formulations, the cumulative exposure to flow agents and fillers compounds significantly. Choosing clean-label for your core supplements—particularly those taken for gut health—limits total excipient burden.
Practical Tips: Transitioning to Clean-Label Supplements
If you're convinced that eliminating flow agents and fillers makes sense, here's how to make the transition practically:
Start with Your Core Supplements: You don't need to replace everything immediately. Begin with supplements you take daily for essential health functions—probiotics for gut health, multivitamins for nutritional insurance, or specialized formulations for specific conditions. These are where excipient exposure is highest and where clean-label benefits matter most.
Read Labels Systematically: Make label reading habitual rather than occasional. Before purchasing any supplement, examine both the Supplement Facts panel and the "Other Ingredients" list. If the excipient list is longer than the active ingredients, that's a red flag.
Research Brands Thoroughly: Look beyond marketing claims to evaluate company philosophy and formulation practices. Companies truly committed to clean labels will explain why they avoid excipients, detail their manufacturing approaches, and provide transparency around sourcing. Marketing fluff like "natural" or "pure" without specifics often masks conventional formulations.
Expect Initial Adjustment: When transitioning from conventional to clean-label probiotics, some people experience temporary digestive changes as gut bacteria adjust to new strains and prebiotic fibers. This is normal and typically resolves within 1-2 weeks. Learn more about optimizing probiotic timing.
Budget Appropriately: Clean-label supplements cost more. Budget $50-100 monthly for high-quality core supplements rather than $20-40 for conventional products. The investment pays dividends in ingredient quality and therapeutic potential.
Monitor Results: Track how you feel after switching to clean-label formulations. Many people report improved digestion, more consistent energy, and better overall wellness when eliminating excipient burden. Keep notes to evaluate whether the premium investment delivers tangible benefits for you personally.
Combine with Lifestyle: Remember that even the best supplements work synergistically with healthy lifestyle choices. Premium probiotics deliver maximum benefit when combined with fiber-rich nutrition, adequate hydration, quality sleep, stress management, and regular physical activity.
The Future of Clean-Label Supplements
Market trends and consumer preferences suggest clean-label formulation is transitioning from niche positioning to mainstream expectation:
Regulatory Momentum: The March 2025 announcement eliminating self-affirmed GRAS, combined with international regulatory action (EU titanium dioxide ban, FDA talc testing requirements), signals increasing scrutiny of supplement excipients. This regulatory tightening will likely accelerate over the next 5-10 years. [15]
Technology Enablement: Advanced manufacturing technologies—blockchain supply chain tracking, QR-linked batch testing, sophisticated capsule materials, precision microencapsulation—make clean-label production increasingly practical. As these technologies mature and costs decline, clean formulation becomes more accessible.
Consumer Education: Growing awareness of excipient impacts, driven by independent research, media coverage, and transparent brand communication, empowers consumers to demand better. The 81% of shoppers who consider clean labels important represents a market force manufacturers cannot ignore. [23]
Competitive Pressure: As premium brands demonstrate clean-label viability and capture market share among educated consumers, mainstream manufacturers face pressure to reformulate. The gap between clean premium ($50-60) and conventional mass-market ($15-30) pricing creates opportunity for mid-tier brands bridging quality and accessibility.
Scientific Validation: Emerging research on excipient impacts—silicon dioxide intestinal damage, microbiome modulation, nanoparticle toxicity—provides scientific foundation for consumer concerns. As evidence accumulates, "clean label" transitions from preference to evidence-based choice.
The trajectory is clear: supplements will gradually move toward cleaner formulations with fewer excipients, greater transparency, and maximum ingredient density. Brands positioned early in this evolution establish competitive advantages—authentic commitment to quality rather than reactive reformulation.
Conclusion: Every Ingredient Should Earn Its Place
Flow agents and fillers exist primarily to solve manufacturer problems, not consumer health challenges. While they enable high-speed production, reduce costs, and extend shelf life, these benefits accrue to producers and retailers rather than the people actually taking supplements daily.
The scientific evidence on flow agent safety presents a nuanced picture. Some excipients like magnesium stearate appear generally benign at typical doses, though concerns about bioavailability impacts and microbiome effects persist. Others like silicon dioxide demonstrate measurable harm to intestinal structure and function at realistic exposure levels. Still others like talc carry contamination risks (asbestos) that no amount of regulatory assurance can fully eliminate.
But perhaps the most compelling argument against excipients isn't direct toxicity—it's wasted opportunity. Every milligram of flow agents, fillers, and lubricants represents a milligram unavailable for beneficial probiotic strains, prebiotic fibers, trace minerals, or botanical extracts. Over months and years of daily supplementation, this opportunity cost compounds dramatically.
Clean-label formulations like MicroBiome Restore prove that alternatives exist. While clean manufacturing requires accepting higher costs, slower production speeds, and more complex quality control, the result is products where literally every ingredient serves therapeutic purpose. From the 26 probiotic strains to the organic sea vegetables to the pullulan capsule itself—nothing is included merely for manufacturing convenience.
This "all-in" philosophy reflects a fundamental question about supplement quality: are we formulating to maximize health benefits or profit margins? The answer should be obvious, yet the prevalence of excipient-laden products suggests many manufacturers prioritize the latter.
As consumers become more educated about excipients and demand greater transparency, the market will continue evolving toward cleaner formulations. But you don't need to wait for industry-wide change. By scrutinizing labels, questioning every ingredient's purpose, and supporting brands committed to purity over profit, you accelerate the transition while ensuring your own supplements deliver maximum therapeutic value rather than minimum acceptable quality.
Your gut health is too important to compromise with wood pulp, machinery lubricants, and silicon dioxide. Choose formulations where every ingredient earns its place through beneficial purpose rather than manufacturing convenience.
For comprehensive gut health support without any fillers or flow agents, explore our Gut Essentials Protocol combining MicroBiome Restore with X-Cellerator Full Spectrum Minerals.
References
- Patel, S., Kaushal, A. M., & Bansal, A. K. (2006). "Compression physics in the formulation development of tablets." Critical Reviews in Therapeutic Drug Carrier Systems, 23(1), 1-65. https://pubmed.ncbi.nlm.nih.gov/16802783/
- Tan, L., & Newton, J. M. (1990). "Powder flowability as an indication of capsule filling performance." International Journal of Pharmaceutics, 61(1-2), 145-155. https://www.sciencedirect.com/science/article/abs/pii/0378517390900537
- European Food Safety Authority (EFSA). (2018). "Re-evaluation of silicon dioxide (E 551) as a food additive." EFSA Journal, 16(1), e05088. https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5088
- Guo, Z., et al. (2018). "Silicon dioxide nanoparticle exposure affects small intestine function in an in vitro model." Nanotoxicology, 12(5), 485-508. https://pmc.ncbi.nlm.nih.gov/articles/PMC6157813/
- Ertel, K. D., & Carstensen, J. T. (1988). "Chemical, physical and lubricant properties of magnesium stearate." Journal of Pharmaceutical Sciences, 77(7), 625-629. https://pubmed.ncbi.nlm.nih.gov/3184259/
- Li, M., et al. (2017). "Magnesium stearate, a widely-used food additive, exhibits a lack of in vitro and in vivo genotoxic potential." Toxicology Reports, 4, 554-560. https://pmc.ncbi.nlm.nih.gov/articles/PMC5655391/
- Vitacost Blog. (2023). "A Guide to Excipients in Vitamins and Supplements." https://www.vitacost.com/blog/excipients-in-vitamins-and-supplements/
- Qin, P., et al. (2021). "High stearic acid diet modulates gut microbiota and aggravates acute graft-versus-host disease." Signal Transduction and Targeted Therapy, 6(1), 228. https://www.nature.com/articles/s41392-021-00600-9
- Thoorens, G., et al. (2014). "Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review." International Journal of Pharmaceutics, 473(1-2), 64-72. https://www.intechopen.com/chapters/68199
- European Food Safety Authority (EFSA). (2018). "Re-evaluation of microcrystalline cellulose as a food additive." EFSA Journal. https://www.efsa.europa.eu/en/efsajournal
- Madan, A. K. (2010). "Key considerations in capsule filling." Pharmaceutical Technology. https://www.pharmtech.com/view/key-considerations-capsule-filling-0
- Vivion. (2022). "Pullulan Capsules 101: The Essential Guide for Manufacturers." https://vivion.com/pullulan-capsules-101-the-essential-guide-for-manufacturers/
- U.S. Food and Drug Administration. (2024). "Generally Recognized as Safe (GRAS)." https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras
- Neltner, T. G., et al. (2013). "Conflicts of interest in approvals of additives to food determined to be generally recognized as safe." JAMA Internal Medicine, 173(22), 2032-2036. https://pubmed.ncbi.nlm.nih.gov/23925667/
- Epstein Becker Green. (2024). "The End of the Self-Affirmed GRAS Pathway?" Health Law Advisor. https://www.healthlawadvisor.com/the-end-of-the-self-affirmed-gras-pathway
- European Food Safety Authority (EFSA). (2021). "Titanium dioxide: E171 no longer considered safe when used as a food additive." https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive
- Wang, J., et al. (2020). "Impact of magnesium stearate presence and variability on drug apparent solubility based on drug physicochemical properties." Pharmaceutics, 12(4), 337. https://pmc.ncbi.nlm.nih.gov/articles/PMC7242257/
- Superior Supplement Manufacturing. (2023). "Capsule Size Chart - Updated Guide." https://www.superiorsupplementmfg.com/blog/capsule-size-chart/
- Supplement Factory UK. (2018). "What Are Excipients? Manufacturing Guide." https://supplementfactoryuk.com/blog/2018/07/what-are-excipients/
- Pharma Excipients. (2021). "The Stability of Probiotics in Pharmaceutical Products." https://www.pharmaexcipients.com/news/stability-probiotics/
- U.S. Food and Drug Administration. (2024). "Questions and Answers on Dietary Supplements." https://www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements
- Li, W., et al. (2019). "Pullulan nanoparticles as prebiotics enhance the antibacterial properties of Lactobacillus plantarum through the induction of mild stress in probiotics." Frontiers in Microbiology, 10, 142. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2019.00142/full
- Natural Products INSIDER. (2024). "Understanding Clean-Label Dietary Supplements." https://www.naturalproductsinsider.com/contract-manufacturing/understanding-clean-label-dietary-supplements
- Egilman, D. S., et al. (2021). "A review of the talc industry's influence on federal regulation and scientific standards for asbestos in talc." American Journal of Public Health, 111(7), 1260-1268. https://pmc.ncbi.nlm.nih.gov/articles/PMC8261788/
- Chan, L. W., et al. (2018). "Elastic recovery of filler-binders to safeguard viability of Lactobacillus rhamnosus GG during direct compression." European Journal of Pharmaceutics and Biopharmaceutics, 131, 79-87. https://www.sciencedirect.com/science/article/abs/pii/S0939641118310828
- Across Biotech. (2023). "HPMC vs Pullulan: Which Is Better For Capsules?" https://acrossbiotech.com/hpmc-vs-pullulan-which-is-better-for-capsules/
- Burgain, J., et al. (2011). "Encapsulation of probiotic living cells: From laboratory scale to industrial applications." Journal of Food Engineering, 104(4), 467-483. https://pmc.ncbi.nlm.nih.gov/articles/PMC10745938/




Share and get 15% off!
Simply share this product on one of the following social networks and you will unlock 15% off!