Probiotic Strains Guide: Benefits and Best Multi-Strain Combos
Think of your gut like a thriving ecosystem—similar to a rainforest. Just as a rainforest needs diverse plant and animal species to stay healthy and resilient, your gut microbiome thrives on bacterial diversity. A single tree species can't create a rainforest, and likewise, a single probiotic strain can't recreate the complex, diverse environment your gut needs to function optimally.
The science is clear: each probiotic strain has specialized functions, much like different workers in a factory performing distinct jobs. Some strains excel at producing digestive enzymes, others strengthen your gut barrier, while others focus on immune modulation or producing vital nutrients. This specialization means that comprehensive gut health requires multiple strains working together—not just one or two doing all the work.
In this guide, you'll discover exactly what makes each probiotic strain unique, why multi-strain formulations mirror the natural diversity of a healthy gut, and how strategic combinations create synergistic effects that single strains simply cannot achieve. We'll explore the 26 clinically-studied strains in MicroBiome Restore and the peer-reviewed research supporting their complementary actions.
🔑 Key Takeaways
Gut Diversity = Gut Health
Healthy microbiomes are characterized by high bacterial diversity. Multi-strain formulations (15-30 strains) better mirror this natural diversity than minimal-strain products, supporting broader metabolic functions and ecosystem resilience.[1]
Strain-Specific Benefits
Each probiotic strain has unique, documented benefits. B. infantis excels for IBS, L. rhamnosus for antibiotic-associated diarrhea, B. breve for weight management—you can't get all benefits from a single strain.[2]
Three Genera = Complete Coverage
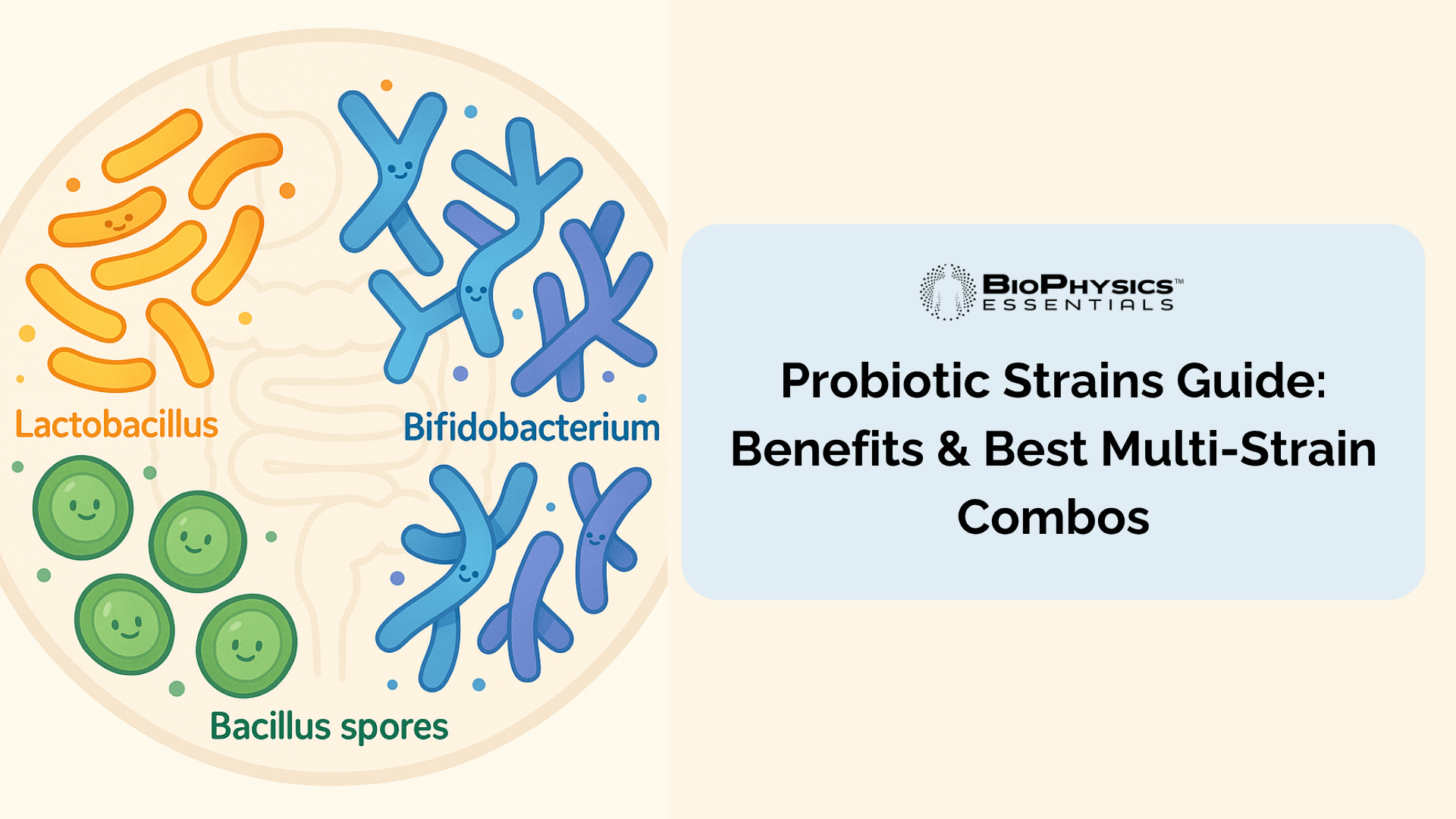
Combining Lactobacillus (small intestine), Bifidobacterium (colon), and Bacillus (acid-resistant spores) provides comprehensive digestive tract coverage that single-genus formulas cannot achieve.[3]
Cross-Feeding Synergy
Different strains feed each other through metabolic cross-feeding. B. bifidum breaks down fibers that L. reuteri uses for growth—these cooperative relationships enhance overall probiotic effectiveness.[4]
Spore Advantage
Soil-based organisms (Bacillus species) survive stomach acid at 90-99% rates versus 30-50% for traditional probiotics, ensuring viable delivery to your intestines where they're needed.[5]
Functional Redundancy
Multi-strain formulations provide backup systems—if one strain doesn't colonize well in your unique gut environment, others with similar functions can compensate, increasing likelihood of benefits.[6]
Understanding Probiotic Strain Specificity: Why Not All Probiotics Are Created Equal
Here's something most people don't realize: the difference between probiotic strains is more significant than the difference between dog breeds. Just as you wouldn't expect a Chihuahua and a Great Dane to have the same abilities despite both being dogs, you can't expect Lactobacillus rhamnosus GG and Lactobacillus acidophilus to provide identical benefits despite both being Lactobacillus species.
What Does "Strain-Specific" Really Mean?
When scientists say probiotic effects are "strain-specific," they mean that each unique bacterial strain has its own genetic blueprint determining exactly what it can do in your body. A systematic review analyzing 228 randomized controlled trials found that 70% of probiotic strains showed significant health benefits—but only when studied individually, not when lumped together by genus or species.[2]
Think of it this way: Lactobacillus rhamnosus GG reduces antibiotic-associated diarrhea by 49%,[7] while Lactobacillus helveticus shows no benefit for the same condition.[2] Both are Lactobacillus species, both are considered probiotics, yet their clinical effects couldn't be more different.
This strain specificity creates both a challenge and an opportunity. The challenge? You need to know exactly which strains you're getting and what they actually do. The opportunity? By strategically combining strains with complementary functions, you can address multiple aspects of gut health simultaneously—something a single strain product simply cannot accomplish.
The Science Behind Gut Microbiome Diversity

Your gut microbiome contains trillions of bacteria representing hundreds of different species. This isn't random—this diversity is essential for your health. Research consistently shows that reduced bacterial diversity is associated with numerous health conditions including inflammatory bowel disease, obesity, type 2 diabetes, and even neurological disorders.[1]
Why Diversity Matters for Your Gut
Imagine your gut as a complex factory with many different production lines. Some bacteria specialize in:
- Breaking down complex carbohydrates that your own enzymes can't digest
- Producing short-chain fatty acids that feed your intestinal cells and reduce inflammation
- Synthesizing vitamins like vitamin K2, folate, and B vitamins
- Training your immune system to distinguish between harmful invaders and harmless substances
- Creating neurotransmitters that influence your mood and brain function
- Maintaining the gut barrier that keeps toxins out of your bloodstream
No single bacterial species can perform all these functions efficiently. Just as a factory needs workers with different specializations, your gut needs bacterial diversity to maintain optimal function.
💡 The Diversity Principle: A probiotic formulation with 20-30 strategically selected strains better mirrors the functional diversity of a healthy gut microbiome than a product containing only 1-5 strains, even at high CFU counts.
Multi-Strain Formulations and Microbiome Restoration
When your gut microbiome becomes disrupted—through antibiotics, poor diet, stress, or illness—you lose both bacterial diversity and function. Research on microbiome restoration reveals that multi-strain probiotic formulations can help reestablish this lost diversity more effectively than single-strain approaches.[8]
When your microbiome has been disrupted by antibiotics, strategic strain selection becomes even more critical—our antibiotic recovery guide covers the 6 clinically proven strains for restoring gut balance.
A clinical study on infants born via C-section (who miss out on vaginal microbiome seeding) found that a multi-strain synbiotic containing Lactobacillus rhamnosus, Bifidobacterium breve, and Propionibacterium with prebiotic fiber successfully corrected microbiome alterations, increased beneficial Bifidobacterium populations, and reduced potentially harmful bacteria.[9]
The key insight? The multi-strain approach promoted beneficial bacteria diversity while simultaneously reducing harmful bacterial overgrowth—creating an environment where multiple beneficial species could thrive together.
The Three Pillars of Complete Probiotic Coverage
A truly comprehensive probiotic formulation combines three distinct bacterial categories, each serving specialized roles in your digestive system. Think of this as three different teams, each working in different areas of your gut to provide complete coverage.
| Bacterial Type | Primary Location | Key Advantages | Stomach Acid Survival |
|---|---|---|---|
| Lactobacillus Species | Small Intestine | Immediate metabolic activity, lactic acid production, immune modulation | 30-50% (varies by strain) |
| Bifidobacterium Species | Large Intestine/Colon | SCFA production, gut barrier support, dominant in healthy infant guts | 30-50% (varies by strain) |
| Bacillus Species (SBOs) | Entire GI Tract | Superior acid resistance, enzyme production, shelf stability | 90-99% |
Why You Need All Three Categories
Each bacterial category colonizes different regions of your digestive tract and performs distinct functions. A formulation containing only Lactobacillus strains, for example, primarily benefits your small intestine but provides limited support for your colon where 70% of your immune system resides.[10]
Lactobacillus species are the rapid responders of your gut. They quickly ferment carbohydrates into lactic acid, creating an acidic environment that discourages pathogenic bacteria while stimulating your immune system. They excel at producing antimicrobial compounds and have been extensively studied for preventing antibiotic-associated diarrhea and supporting vaginal health.[7]
Bifidobacterium species are the foundation builders. Dominant in healthy infant guts (making up 60-90% of gut bacteria in breastfed babies), they produce short-chain fatty acids that feed your intestinal cells, strengthen your gut barrier, and powerfully modulate your immune system. They're particularly effective for IBS, inflammatory conditions, and metabolic health.[11]
Bacillus species (soil-based organisms) are the survivors. Encased in protective spore coats, they pass through stomach acid almost completely intact—a revolutionary advantage over traditional probiotics that lose 50-70% of their population before reaching your intestines. Once in the gut, they germinate and produce powerful digestive enzymes and antimicrobial compounds.[5]

The Synergy Effect: Research shows that combining strains from different genera creates complementary effects. Lactobacillus species produce lactic acid that lowers pH, creating an environment where Bifidobacterium species thrive. Bifidobacterium species produce acetate that other bacteria convert to butyrate—your intestinal cells' primary fuel source. This metabolic cooperation is impossible with single-genus formulations.[4]
Soil-Based Organisms: The Revolutionary Probiotic Advantage
Here's a game-changing fact that most people don't know: traditional Lactobacillus and Bifidobacterium probiotics lose 50-70% of their bacteria during passage through your stomach acid. This means if you take a capsule with 10 billion CFU, only 3-5 billion might actually reach your intestines alive.[5]
Soil-based organisms (SBOs) change this equation completely.
What Makes SBOs Different?
Bacillus species are spore-forming bacteria, meaning they can create a protective armor called an endospore. Think of it like a seed—it can survive harsh conditions (heat, cold, acid, lack of moisture) and then "wake up" when conditions are right. In your digestive system, this means:
- 90-99% survival through stomach acid compared to 30-50% for traditional probiotics[5]
- No refrigeration required—spores remain stable at room temperature indefinitely
- Germination in the small intestine—they activate precisely where they're needed
- Powerful enzyme production—proteases and amylases that enhance nutrient absorption
- Unique antimicrobial compounds—lipopeptides that target pathogens while sparing beneficial bacteria
Bacillus coagulans: The Clinical Heavyweight
This well-researched SBO reduces IBS abdominal pain by clinically significant margins (p<0.001), enhances protein absorption by 20% including branch-chain amino acids critical for muscle health, and demonstrates measurable weight reduction in overweight individuals.[12] Unlike traditional probiotics, B. coagulans produces lactic acid while in spore form, making it uniquely effective even before germination.
Bacillus subtilis: The Gut Reconditioner
This strain doesn't try to permanently colonize your gut—instead, it reconditions your microbiome environment. It produces antimicrobial peptides that suppress pathogens while secreting growth factors that encourage your own beneficial bacteria to flourish. Clinical trials show it reduces bloating and burping in 47.4% of subjects compared to 22.2% with placebo.[13]
Bacillus clausii: The Antibiotic-Resistant Ally
Here's something remarkable: B. clausii possesses natural resistance to many antibiotics, meaning you can take it during antibiotic therapy to protect your gut microbiome. It shortens persistent diarrhea recovery by 2 days and produces antimicrobial compounds active against C. difficile and S. aureus.[14]
A groundbreaking study comparing spore-based probiotics to traditional strains found that spore-based formulations achieved a 42% reduction in post-meal endotoxins and 24% reduction in triglycerides—benefits not seen with non-spore-forming probiotics.[5] The researchers noted that while Bifidobacterium longum and B. breve became undetectable within one hour in simulated gastric juice, spores reached the intestines 100% viable.
Meet the 26 Strains: Your Complete Probiotic Team
MicroBiome Restore contains 26 clinically-studied probiotic strains, each selected for specific, documented benefits. Rather than randomly combining strains, this formulation strategically includes complementary bacteria that work together to support comprehensive gut health.
The Bifidobacterium Brigade (5 Strains): Your Colon's Best Friends
Bifidobacterium species are your colon's primary beneficial bacteria, dominant in healthy infant guts and essential for immune development and gut barrier function.
Bifidobacterium bifidum
Specialty: IBS Relief & Gut Barrier Strengthening
Clinical trials show B. bifidum reduces IBS symptoms by 47% versus 11% placebo through upregulating tight junction proteins and superior adhesion to intestinal cells.[15] It's also an "extracellular degrader" that breaks down complex fibers, releasing nutrients that feed other beneficial bacteria—a key cross-feeding role.
Bifidobacterium breve
Specialty: Weight Management & Mental Health
The B-3 strain demonstrates remarkable anti-obesity effects with significant body fat reduction in clinical trials.[16] It also shows mental health benefits, reducing depression scores while supporting preterm infant gut development. This strain excels at producing anti-inflammatory compounds and modulating the gut-brain axis.
Bifidobacterium infantis
Specialty: IBS Gold Standard & Immune Support
Considered the premier probiotic for IBS, B. infantis 35624 is uniquely adapted to metabolize human milk oligosaccharides and produces acetate as the primary infant gut SCFA.[17] Studies show significant improvements in abdominal pain, bloating, and bowel movement difficulty in women with IBS.
Bifidobacterium lactis
Specialty: Cardiovascular Health & Constipation Relief
This strain improves cardiovascular outcomes when combined with statins and reduces colonic transit time in constipation—meaning faster, more comfortable bowel movements.[18] It modulates the gut-heart axis through beneficial metabolite production and has been shown to reduce transit time from 50 hours to 30 hours in clinical studies.
Bifidobacterium longum
Specialty: Sleep Quality, Cognitive Function & Mood
B. longum 1714 improves sleep quality and cognitive function in elderly populations. It produces multiple short-chain fatty acids including acetate, propionate, and butyrate, and systematically alters gut composition to support mental wellness.[19] This strain represents one of the most well-studied "psychobiotics" influencing the gut-brain axis.
The Lactobacillus Legion (10 Strains): Your Small Intestine Specialists
Lactobacillus species primarily colonize the small intestine, producing antimicrobial compounds and modulating immune responses through multiple pathways.
Lactobacillus acidophilus
Specialty: Pain Relief & Digestive Support
This strain induces μ-opioid and cannabinoid receptors, providing morphine-like analgesic effects for IBS pain—a unique mechanism among probiotics.[20] The NCFM strain has been studied in over 45 clinical trials, demonstrating benefits for lactose intolerance, diarrhea prevention, and immune enhancement.
Lactobacillus casei
Specialty: Immune Enhancement & Infection Prevention
L. casei increases natural killer cell activity and interferon-γ production, reducing respiratory infection incidence.[21] The Shirota strain is consumed by 2.4 million people daily in Yakult probiotic beverages, with decades of safety data and documented immune benefits.
Lactobacillus delbrueckii subsp. bulgaricus
Specialty: Immune Stimulation & Longevity
This yogurt culture strain produces immunostimulatory exopolysaccharides that enhance influenza vaccine response and reduce common cold risk in elderly populations.[22] It's associated with Bulgarian population longevity and works synergistically with Streptococcus thermophilus in fermented dairy products.
Lactobacillus fermentum
Specialty: Blood Sugar Control & Cognitive Function
L. fermentum demonstrates anti-diabetic effects by reducing fasting blood glucose while improving cognitive function in aging studies.[23] It also excels at restoring vaginal flora and shows superior adherence to intestinal cells compared to many other Lactobacillus species.
Lactobacillus gasseri
Specialty: Visceral Fat Reduction
This strain specifically targets abdominal fat, reducing visceral adipose tissue by 4.6% through upregulating fatty acid oxidation genes.[24] Clinical trials show measurable decreases in waist circumference, body weight, and BMI with 12 weeks of supplementation.
Lactobacillus paracasei
Specialty: Immune Support & Anti-Inflammatory Effects
L. paracasei increases vaccine-specific IgG levels and produces anti-inflammatory extracellular vesicles that reduce cytokine expression.[25] It also serves as a "keystone strain" for prebiotic degradation, breaking down inulin and releasing nutrients that feed other beneficial bacteria.
Lactobacillus plantarum
Specialty: IBS, Constipation & Cardiovascular Health
The 299v strain shows superior mucosa attachment through mannose-binding adhesion, enabling prolonged gut colonization.[26] It improves IBS symptoms, relieves constipation, and reduces cardiovascular inflammation markers in multiple clinical studies.
Lactobacillus reuteri
Specialty: Infant Colic & Bone Health
L. reuteri reduces infant colic crying time dramatically and produces reuterin, a broad-spectrum antimicrobial compound.[27] Remarkably, it also regulates bone mass density and synthesizes vitamin B12 and folate—unique benefits among probiotics.
Lactobacillus rhamnosus
Specialty: Antibiotic-Associated Diarrhea Prevention
L. rhamnosus GG is the most widely studied probiotic worldwide (800+ published studies) and reduces antibiotic-associated diarrhea by 49%.[7] It expresses unique pili structures for superior adhesion and demonstrates benefits across diverse conditions from eczema to respiratory infections.
Lactobacillus salivarius
Specialty: Oral Health & Pathogen Elimination
This strain reduces halitosis and periodontitis through hydrogen peroxide and bacteriocin production.[28] Remarkably, it achieves complete killing of MRSA (antibiotic-resistant staph) strains in 24-hour co-culture—a powerful antimicrobial capability.
The Bacillus Battalion (5 Strains): Your Acid-Resistant Guardians
These soil-based organisms provide revolutionary survivability advantages through protective spore coats, ensuring viable delivery throughout your entire digestive tract.
Bacillus coagulans
Specialty: IBS Relief & Protein Absorption
Reduces IBS abdominal pain intensity by clinically significant margins (p<0.001) and enhances protein absorption by 20%, including branch-chain amino acids.[12] It also demonstrates significant weight reduction effects in overweight individuals through gut microbiota modulation.
Bacillus clausii
Specialty: Diarrhea Recovery & Antibiotic Resistance
Shortens persistent diarrhea recovery by 2 days and produces clausin antimicrobials active against C. difficile and S. aureus.[14] Its unique antibiotic resistance allows safe co-administration during antibiotic therapy to protect gut health.
Bacillus subtilis
Specialty: Bloating Relief & Microbiome Reconditioning
Improves bloating and burping in 47.4% of subjects versus 22.2% placebo while increasing anti-inflammatory immune populations.[13] Consumed safely for centuries in natto (Japanese fermented soybeans), it reconditions gut environment rather than attempting permanent colonization.
Bacillus licheniformis
Specialty: Stress-Related Gut Recovery
Recovers stress-destroyed gut microbiota balance and produces antimicrobial peptides with strong bactericidal capacity.[29] It induces macrophage extracellular traps that decrease S. aureus colonies, providing immune system support alongside microbiome benefits.
Bacillus pumilus
Specialty: Inflammatory Bowel Support
Significantly improves colitis disease activity index and reduces inflammatory cell infiltration in research models.[30] It demonstrates immunostimulatory effects through CD4+CD8+ T cell activation, making it valuable for inflammatory gut conditions.
The Complementary Crew (3 Strains): Unique Mechanisms for Complete Coverage
Streptococcus thermophilus
Specialty: Lactose Digestion & Immune Modulation
Produces exceptionally high beta-galactosidase levels for lactose metabolism and increases folate content in fermented foods up to 20-fold.[31] It reduces inflammatory markers (TNF-α, IL-1β, IL-6) while enhancing Th1 immune response, working symbiotically with L. bulgaricus.
Lactococcus lactis subsp. lactis
Specialty: Immune Stimulation & Antimicrobial Production
Achieves remarkable immunostimulatory capacity—the CAB701 strain reaches 86.3% of LPS-induced nitric oxide production versus L. rhamnosus GG at 18.5%.[32] It produces nisin bacteriocins that inhibit Listeria, E. coli, Salmonella, and Staphylococcus.
Saccharomyces boulardii
Specialty: Antibiotic-Associated Diarrhea & C. difficile
The only beneficial yeast in the formulation, S. boulardii secretes a protease that digests C. difficile toxins while blocking inflammatory pathways.[33] Meta-analysis of 31 trials (5,029 patients) showed significant efficacy in 84% of treatment arms for antibiotic-associated diarrhea, travelers' diarrhea, and IBS.
Cross-Feeding and Metabolic Synergy: How Strains Work Together
Here's where multi-strain formulations truly shine: different bacterial strains can feed each other through metabolic cross-feeding relationships, creating cooperative networks that enhance overall effectiveness. This isn't speculation—it's documented in peer-reviewed research.

Real-World Examples of Bacterial Cooperation
Think of cross-feeding like a relay race where one runner passes the baton to the next. In your gut, one bacterial strain breaks down a complex fiber into simpler compounds, which another strain then uses for energy and growth.
Example #1: The Bifidobacterium-Lactobacillus Partnership
Bifidobacterium breve produces 1,2-propanediol from fucose metabolism. Lactobacillus reuteri can't make this compound itself, but it thrives when it's available. Gnotobiotic mouse studies proved this cross-feeding relationship enhances L. reuteri's competitive fitness in the gut.[4] The researchers concluded this partnership "could be considered in generating probiotic products by pairing L. reuteri with Bifidobacterium species."
Example #2: The Fiber-Breaking Team
Bifidobacterium bifidum acts as an "extracellular degrader"—it breaks down complex human milk oligosaccharides outside its cell, releasing lactose, fucose, and sialic acid into the gut environment. Other Bifidobacterium species (like B. infantis and B. longum) that can't break down these complex sugars efficiently then use these released compounds for their own growth.[34] Without B. bifidum doing the initial breakdown work, these other beneficial species would struggle.
Example #3: The Acetate-to-Butyrate Conversion
Bifidobacterium species produce acetate as a primary metabolic byproduct. Other bacteria like Faecalibacterium prausnitzii then convert this acetate into butyrate—the preferred fuel source for your colon cells that provides 70% of their energy.[35] This two-step process requires bacterial diversity; neither species alone can complete the full pathway efficiently.
Why This Matters for Your Gut Health
These cross-feeding relationships mean that the whole is greater than the sum of its parts. A formulation with 20+ strains creates a network of metabolic cooperation that mimics the natural complexity of a healthy gut microbiome. Single-strain or minimal-strain products simply cannot recreate these cooperative relationships.
Research on rational probiotic combination design found that combining Bacteroides xylanisolvens with Clostridium butyricum created bidirectional cross-feeding (each feeds the other) and showed superior therapeutic efficacy against metabolic disorders in obese mice compared to either strain alone.[36] This led to development of next-generation live biotherapeutic products based on these synergistic principles.
Functional Categories: How Different Strains Support Different Aspects of Health
Each probiotic strain falls into one or more functional categories based on its primary mechanisms of action. A comprehensive formulation includes strains across all these categories to provide complete support.
Digestive Enzyme Production
Some strains excel at producing enzymes that help you break down and absorb nutrients:
- Beta-galactosidase for lactose digestion (S. thermophilus, L. acidophilus, Bifidobacterium species)
- Proteases for protein breakdown (S. boulardii, Bacillus species)
- Amylases for carbohydrate digestion (Bacillus species)
- Bile salt hydrolase for fat digestion and cholesterol metabolism (L. plantarum, L. lactis)
Bacillus coagulans, for example, increases amino acid absorption by 20%, including branch-chain amino acids critical for muscle health and recovery.[12]
Immune System Modulation
Your gut houses 70% of your immune system, and different probiotic strains modulate immunity through distinct pathways:
- Cytokine balancing—reducing pro-inflammatory TNF-α, IL-1β, IL-6 while increasing anti-inflammatory IL-10
- Natural killer cell activation—enhancing your first-line defense against viruses and cancer cells (L. casei)
- Antibody production—increasing secretory IgA that protects mucosal surfaces (L. rhamnosus, S. boulardii)
- Dendritic cell maturation—training immune cells to distinguish threats from harmless substances (Bifidobacterium species)
- Regulatory T cell induction—preventing excessive inflammation and autoimmune reactions (multiple strains)
L. lactis CAB701 demonstrates particularly impressive immune activation, achieving 86.3% of LPS-induced nitric oxide production—a marker of immune readiness—compared to just 18.5% for L. rhamnosus GG.[32]
For an evidence-based breakdown of how different L. rhamnosus strains—GG, GR-1, JB-1, and CGMCC1.3724—produce distinct clinical effects, see our dedicated Lactobacillus rhamnosus benefits article.
Gut Barrier Enhancement
Your intestinal barrier prevents toxins and undigested food particles from entering your bloodstream. Multiple probiotic strains strengthen this barrier through complementary mechanisms:
- Tight junction protein upregulation—increasing claudins, occludins, and ZO-1 proteins that seal cell junctions
- Mucin production stimulation—thickening your protective mucus layer
- Epithelial cell proliferation—accelerating gut lining repair and regeneration
- Inflammation reduction—preventing barrier breakdown from chronic inflammation
Research on multi-strain probiotic formulations showed they significantly improved transepithelial electrical resistance (a measure of barrier integrity) while reducing inflammatory markers and increasing beneficial bacterial populations.[37]
Neurotransmitter Production and Mental Health
The gut-brain axis is real, and specific probiotic strains produce or influence neurotransmitters that affect your mood, anxiety, and cognitive function:
- GABA production—the calming neurotransmitter (Lactobacillus and Bifidobacterium species)
- Serotonin pathway modulation—95% of serotonin is produced in the gut
- BDNF enhancement—brain-derived neurotrophic factor supports neuroplasticity and learning
- Stress response regulation—modulating cortisol and inflammatory stress markers
Clinical studies show B. longum 1714 improves sleep quality and well-being in healthy adults,[19] while multi-species probiotics containing both Lactobacillus and Bifidobacterium strains reduce emotional salience and improve mood in subjects with moderate depression.[38]
Pathogen Inhibition and Antimicrobial Protection
Different strains fight harmful bacteria through diverse antimicrobial mechanisms:
- Bacteriocin production—L. lactis produces nisin that kills Listeria, Salmonella, and Staphylococcus
- Organic acid production—lactic acid from Lactobacillus lowers pH to inhibit pathogens
- Hydrogen peroxide generation—oxidative stress that harms pathogenic bacteria
- Competitive exclusion—occupying binding sites and consuming nutrients pathogens need
- Toxin degradation—S. boulardii's protease destroys C. difficile toxins
L. salivarius demonstrates remarkable antimicrobial power, achieving complete killing of MRSA (methicillin-resistant Staphylococcus aureus) in 24-hour co-culture experiments.[28]
Short-Chain Fatty Acid Production
SCFAs are among the most important metabolites produced by gut bacteria, affecting everything from colon health to brain function:
- Butyrate—primary fuel for colon cells, anti-inflammatory, gene expression regulator
- Propionate—regulates gluconeogenesis in liver, provides satiety signaling
- Acetate—crosses blood-brain barrier, substrate for other bacterial metabolism
Bifidobacterium and Lactobacillus species are major SCFA producers, with physiological effects including enhanced gut barrier integrity, improved insulin sensitivity, appetite regulation, and anti-inflammatory signaling throughout the body.[35]
Why Multi-Strain Formulations Outperform Minimal-Strain Products
Now that you understand strain specificity, gut diversity principles, and functional categories, let's address the central question: Why would you choose a 20-30 strain formulation over a product with just 1-5 strains?
Functional Redundancy and Resilience
Your gut microbiome is unique. Research shows that probiotic colonization is highly personalized—some people are "permissive" to certain strains while others show "colonization resistance."[39] When you take a single-strain probiotic, you're hoping that one strain will colonize well in YOUR unique gut environment.
Multi-strain formulations provide functional redundancy—multiple strains capable of similar functions. If one doesn't colonize effectively in your gut, others with complementary capabilities can compensate. Think of it like having backup systems: if one fails, others continue providing benefits.
💡 The Colonization Reality: A landmark Cell study found marked person-to-person differences in probiotic colonization patterns. The native microbiome creates colonization barriers through competitive exclusion, with your existing gut bacteria determining whether supplemented probiotics can establish themselves.[39] Multi-strain formulations increase the likelihood that multiple strains will find their niche in your unique gut ecosystem.
Broader Metabolic Coverage
No single strain performs all beneficial functions efficiently. Consider what a single-strain product cannot do:
- A Lactobacillus-only product won't provide the colon-specific benefits of Bifidobacterium species
- A Bifidobacterium-only product loses 50-70% of bacteria to stomach acid
- Non-spore-forming probiotics can't produce the digestive enzymes Bacillus species provide
- Most bacterial probiotics lack the unique toxin-degrading abilities of S. boulardii
A comprehensive formulation with 20+ strains provides metabolic versatility that single-strain products fundamentally cannot match.

Evidence for Multi-Strain Advantages
While systematic reviews show that more strains don't automatically equal better results,[40] research also demonstrates specific contexts where multi-strain combinations outperform single strains:
- Necrotizing enterocolitis prevention—multiple strains combining Bifidobacterium and Lactobacillus significantly reduced NEC in premature infants[41]
- H. pylori eradication—L. rhamnosus GG combined with B. lactis proved more effective than L. rhamnosus alone[40]
- Antibiotic-associated diarrhea—multi-strain formulations showed significant prevention across 19 RCTs[42]
- Mood and depression—multispecies probiotics with both Bifidobacteria and Lactobacilli appear more effective than single strains[38]
Chapman et al. found that in 75% of cases (12 out of 16 studies), mixtures showed greater effectiveness than single strains, though whether this resulted from synergistic interactions or simply higher total CFU remained debated.[43]
Mimicking Natural Microbiome Diversity
Perhaps the most compelling argument: healthy gut microbiomes are diverse. Reduced bacterial diversity is consistently associated with inflammatory bowel disease, obesity, type 2 diabetes, autism spectrum disorders, and metabolic syndrome.[1]
A probiotic formulation with 20-30 strategically selected strains better approximates the functional diversity of a healthy microbiome than a product with 1-5 strains. While probiotics generally don't permanently colonize (requiring continued supplementation), multi-strain formulations can temporarily restore functional diversity and create lasting ecosystem changes through cross-feeding relationships.[44]
The MicroBiome Restore Advantage: Evidence-Based Formulation
MicroBiome Restore's 26-strain formulation isn't about achieving an impressive strain count—it's about strategic selection based on scientific evidence. Each strain was chosen for documented clinical benefits and complementary functions.
Multi-Genera Diversity for Complete Coverage
The formulation combines three distinct bacterial categories:
- 10 Lactobacillus strains for small intestine colonization, rapid metabolic activity, and antimicrobial protection
- 5 Bifidobacterium strains for colon dominance, SCFA production, and immune modulation
- 5 Bacillus strains (SBOs) for superior acid resistance (90-99% survival), enzyme production, and microbiome reconditioning
- 3 complementary strains (S. thermophilus, L. lactis, S. boulardii) for unique mechanisms unavailable to other categories
This multi-genera approach aligns with research showing that combining strains from different genera creates complementary effects through niche differentiation and metabolic cooperation.[3]
Synbiotic Enhancement with 9 Organic Prebiotics
The formulation includes 9 certified organic prebiotic ingredients that selectively feed beneficial bacteria:
- Norwegian kelp, bladderwrack, oarweed—marine-sourced prebiotics with unique polysaccharides
- Jerusalem artichoke—premier source of inulin, the gold-standard prebiotic
- Fig fruit—natural fiber source with gentle digestive support
- Maitake mushroom—"super prebiotic" stimulating Lactobacillus and Bifidobacterium growth
- Acacia fiber—high soluble fiber promoting SCFA production
- Bioactive leonardite with humic and fulvic acids—providing 80+ trace minerals and bioactive compounds
These prebiotics work synergistically with the probiotic strains, providing substrates for bacterial metabolism and promoting cross-feeding relationships.[45]
Shelf-Stable Pullulan Capsules
The formulation uses pullulan capsules—a fermented, plant-based material that itself functions as a prebiotic. Pullulan provides excellent oxygen barrier properties (protecting probiotic viability), quickly converts to short-chain fatty acids in the gut, and promotes Bifidobacterium and Lactobacillus activity.[46]
How to Choose a Quality Multi-Strain Probiotic
Not all multi-strain formulations are created equal. Here's what to look for when evaluating probiotic supplements:
Strain-Level Identification
The label should list genus, species, AND strain designation for each probiotic. For example:
- ✅ Good: "Lactobacillus rhamnosus GG" or "Bifidobacterium longum 1714"
- ❌ Insufficient: "Lactobacillus rhamnosus" or just "Lactobacillus blend"
Strain designation matters because different strains of the same species have dramatically different effects. Research shows that strain-specific identification is essential for reproducible results and accurate health claims.[2]
CFU Count at Expiration (Not Just Manufacture)
Many products list impressive CFU counts "at time of manufacture" but don't guarantee counts at expiration. Quality products provide:
- CFU guarantees at end of shelf life
- Third-party verification of bacterial counts
- Proper storage requirements clearly stated
- Adequate doses of each individual strain (not just impressive total CFU)
Most clinically effective formulations deliver 25-50 billion CFU per serving, though the specific dose requirements vary by strain.[47]
Evidence-Based Strain Selection
Quality formulations include strains with documented clinical research. Look for:
- Published studies on the specific strains included
- Safety data including regulatory designations (GRAS, QPS)
- Complementary functions rather than redundant strains
- Multi-genera diversity (Lactobacillus + Bifidobacterium + Bacillus)
⚠️ Quality Over Quantity: A formulation with 5-8 well-characterized, clinically-studied strains at adequate doses may prove more effective than 20+ unstudied strains combined without compatibility testing or finished formulation validation.[47]
Manufacturing Quality Standards
Reputable manufacturers provide:
- Good Manufacturing Practices (GMP) certification
- Third-party testing for strain identity and purity
- Contamination testing for pathogens and heavy metals
- Stability testing ensuring viability through shelf life
- Transparent labeling with complete ingredient disclosure
Frequently Asked Questions About Probiotic Strains
Do I need to take probiotics with food?
It depends on the strains. Traditional Lactobacillus and Bifidobacterium strains generally survive better when taken with food (especially fatty meals), as food buffers stomach acid. However, spore-forming Bacillus species survive stomach acid regardless of food intake. For multi-strain formulations containing both types, taking with food is generally recommended for optimal survival of all strains.
How long does it take to see results from probiotics?
Timeline varies by condition and strain:
- Acute diarrhea—improvements within 24-48 hours
- Bloating and gas—noticeable changes in 1-2 weeks
- IBS symptoms—meaningful improvement typically requires 4-8 weeks
- Immune benefits—measurable changes in immune markers at 4-12 weeks
- Mood and cognitive effects—may require 8-12 weeks for noticeable benefits
Consistency matters more than duration for most benefits—probiotics generally provide transient effects requiring continued supplementation.[39]
Can I take probiotics with antibiotics?
Yes, but timing and strain selection matter. L. rhamnosus GG reduces antibiotic-associated diarrhea by 49% when taken during antibiotic therapy.[7] Bacillus clausii possesses natural antibiotic resistance, making it ideal for concurrent use.[14] S. boulardii, being a yeast, isn't affected by antibacterial antibiotics.
Take probiotics at least 2-3 hours away from antibiotic doses to minimize direct killing of probiotic bacteria. Continue probiotics for 2-4 weeks after completing antibiotics to support microbiome recovery.
Will probiotics permanently colonize my gut?
Most supplemented probiotics provide transient benefits without permanent colonization. Research shows that after discontinuing probiotics, most strains clear from stool within days to weeks.[39]
However, probiotics can create lasting ecosystem changes through:
- Cross-feeding relationships that persist after probiotic discontinuation
- Microbiome reconditioning that promotes growth of native beneficial bacteria
- Immune system training with sustained effects on immune function
- Metabolite production that temporarily alters gut environment
For sustained benefits, continued supplementation is generally recommended.
Are more CFUs always better?
Not necessarily. Clinical studies show effective doses ranging from 1 billion to 900 billion CFU depending on strain and condition.[47] Some strains demonstrate effectiveness at relatively low doses (100 million to 1 billion CFU), while others require higher doses (10-50 billion CFU) for clinical benefits.
What matters more than total CFU:
- Strain-specific dosing based on clinical research
- Viability at consumption (guaranteed at expiration, not just manufacture)
- Survival through stomach acid (where SBOs excel)
- Multiple complementary strains providing diverse functions
Conclusion: Building Your Optimal Gut Ecosystem
Your gut microbiome is one of the most complex ecosystems on Earth, containing trillions of bacteria representing hundreds of species. Just as a diverse rainforest is more resilient and productive than a monoculture, a diverse gut microbiome is more resilient, functional, and health-promoting than one dominated by just a few species.
The science is unequivocal: each probiotic strain has specialized, documented functions that cannot be replicated by other strains—even within the same species. Bifidobacterium infantis excels for IBS, Lactobacillus gasseri targets visceral fat, Bacillus coagulans survives stomach acid at 95%+ rates, and Saccharomyces boulardii degrades C. difficile toxins. No single strain performs all these functions.
This is why comprehensive gut health requires a multi-strain approach that:
- Mirrors natural microbiome diversity with 20+ complementary strains
- Combines three bacterial genera (Lactobacillus, Bifidobacterium, Bacillus) for complete digestive tract coverage
- Includes spore-forming organisms with 90-99% acid survival rates
- Creates cross-feeding relationships where strains metabolically support each other
- Provides functional redundancy so benefits occur even if some strains don't colonize well in your unique gut
- Addresses multiple health aspects through diverse mechanisms—digestive, immune, metabolic, neurological, and antimicrobial
MicroBiome Restore's 26-strain formulation represents this evidence-based approach: strategically combining clinically-validated strains with complementary functions, enhanced by 9 organic prebiotics that selectively feed beneficial bacteria and support the cross-feeding networks that make multi-strain formulations truly effective.
Remember: quality beats quantity, but strategic diversity beats minimalism. A well-formulated multi-strain probiotic with 20-30 evidence-based strains provides the functional diversity, metabolic versatility, and colonization resilience that minimal-strain products fundamentally cannot match.
Your gut is an ecosystem. Feed it like one.




Share and get 15% off!
Simply share this product on one of the following social networks and you will unlock 15% off!